|
Available Energy
In thermodynamics, the exergy of a System (thermodynamics), system is the maximum useful Mechanical work, work possible during a Thermodynamic process, process that brings the system into Thermodynamic equilibrium, equilibrium with a heat reservoir, reaching maximum entropy. When the Surroundings (thermodynamics), surroundings are the reservoir, exergy is the potential of a system to cause a change as it achieves equilibrium with its environment. Exergy is the energy that is available to be used. After the system and surroundings reach equilibrium, the exergy is zero. Determining exergy was also the first goal of thermodynamics. The term "exergy" was coined in 1956 by Zoran Rant (1904–1972) by using the Greek ''wikt:ἐξ, ex'' and ''wikt:ἔργον, ergon'' meaning "from Work (thermodynamics), work", but the concept had been earlier developed by Josiah Willard Gibbs, J Willard Gibbs (the namesake of Gibbs free energy) in 1873. Energy is neither created nor destroyed during a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
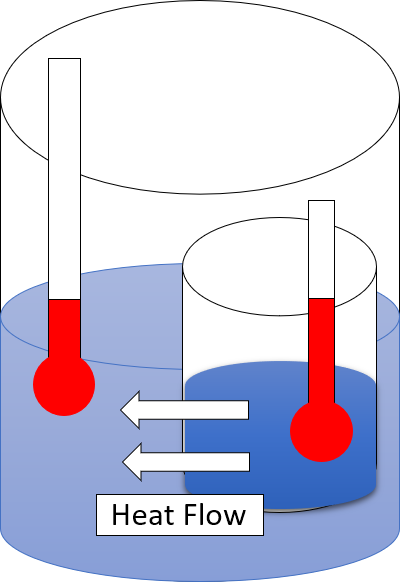
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isolated System
In physical science, an isolated system is either of the following: # a physical system so far removed from other systems that it does not interact with them. # a thermodynamic system enclosed by rigid immovable walls through which neither mass nor energy can pass. Though subject internally to its own gravity, an isolated system is usually taken to be outside the reach of external gravitational and other long-range forces. This can be contrasted with what (in the more common terminology used in thermodynamics) is called a closed system, being enclosed by selective walls through which energy can pass as heat or work, but not matter; and with an open system, which both matter and energy can enter or exit, though it may have variously impermeable walls in parts of its boundaries. An isolated system obeys the conservation law that its total energy–mass stays constant. Most often, in thermodynamics, mass and energy are treated as separately conserved. Because of the require ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Heat Engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates. A heat engine acts by transferring energy from a warm region to a cool region of space and, in the process, converting some of that energy to mechanical work. The cycle may also be reversed. The system may be worked upon by an external force, and in the process, it can transfer thermal energy from a cooler system to a warmer one, thereby acting as a refrigerator or heat pump rather than a heat engine. Every t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
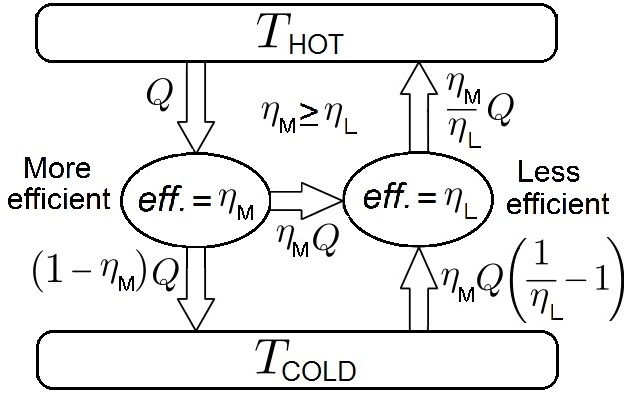
Carnot's Theorem (thermodynamics)
In thermodynamics, Carnot's theorem, developed in 1824 by Nicolas Léonard Sadi Carnot, also called Carnot's rule, is a principle that specifies limits on the maximum efficiency that any heat engine can obtain. Carnot's theorem states that all heat engines operating between the same two thermal or heat reservoirs can't have efficiencies greater than a reversible heat engine operating between the same reservoirs. A corollary of this theorem is that every reversible heat engine operating between a pair of heat reservoirs is equally efficient, regardless of the working substance employed or the operation details. Since a Carnot heat engine is also a reversible engine, the efficiency of all the reversible heat engines is determined as the efficiency of the Carnot heat engine that depends solely on the temperatures of its hot and cold reservoirs. The maximum efficiency (i.e., the Carnot heat engine efficiency) of a heat engine operating between cold and hot reservoirs, denoted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag. In general, an engine is any machine that converts energy to mechanical work. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reversible Computing
Reversible computing is any model of computation where the computational process, to some extent, is time-reversible. In a model of computation that uses deterministic transitions from one state of the abstract machine to another, a necessary condition for reversibility is that the relation of the mapping from states to their successors must be one-to-one. Reversible computing is a form of unconventional computing. Due to the unitarity of quantum mechanics, quantum circuits are reversible, as long as they do not " collapse" the quantum states they operate on. Reversibility There are two major, closely related types of reversibility that are of particular interest for this purpose: physical reversibility and logical reversibility. A process is said to be ''physically reversible'' if it results in no increase in physical entropy; it is isentropic. There is a style of circuit design ideally exhibiting this property that is referred to as charge recovery logic, adiabatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Information Theory
Information theory is the scientific study of the quantification, storage, and communication of information. The field was originally established by the works of Harry Nyquist and Ralph Hartley, in the 1920s, and Claude Shannon in the 1940s. The field is at the intersection of probability theory, statistics, computer science, statistical mechanics, information engineering, and electrical engineering. A key measure in information theory is entropy. Entropy quantifies the amount of uncertainty involved in the value of a random variable or the outcome of a random process. For example, identifying the outcome of a fair coin flip (with two equally likely outcomes) provides less information (lower entropy) than specifying the outcome from a roll of a die (with six equally likely outcomes). Some other important measures in information theory are mutual information, channel capacity, error exponents, and relative entropy. Important sub-fields of information theory include s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Function
In the thermodynamics of equilibrium, a state function, function of state, or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities (that describe equilibrium states of a system) that depend only on the current equilibrium thermodynamic state of the system (e.g. gas, liquid, solid, crystal, or emulsion), not the path which the system has taken to reach that state. A state function describes equilibrium states of a system, thus also describing the type of system. A state variable is typically a state function so the determination of other state variable values at an equilibrium state also determines the value of the state variable as the state function at that state. The ideal gas law is a good example. In this law, one state variable (e.g., pressure, volume, temperature, or the amount of substance in a gaseous equilibrium system) is a function of other state variables so is regarded as a state function. A stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinetic energy. It keeps account of the gains and losses of energy of the system that are due to changes in its internal state. It does not include the kinetic energy of motion of the system as a whole, or any external energies from surrounding force fields. The internal energy of an isolated system is constant, which is expressed as the law of conservation of energy, a foundation of the first law of thermodynamics. The internal energy is an extensive property. The internal energy cannot be measured directly and knowledge of all its components is rarely interesting, such as the static rest mass energy of its constituent matter. Thermodynamics is chiefly concerned only with ''changes'' in the internal energy, not with its absolute value. Inste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule
The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force applied. It is also the energy dissipated as heat when an electric current of one ampere passes through a resistance of one ohm for one second. It is named after the English physicist James Prescott Joule (1818–1889). Definition In terms of SI base units and in terms of SI derived units with special names, the joule is defined as One joule can also be defined by any of the following: * The work required to move an electric charge of one coulomb through an electrical potential difference of one volt, or one coulomb-volt (C⋅V). This relationship can be used to define the volt. * The work required to produce one watt of power for one second, or one watt-second (W⋅s) (compare kilowatt-hour, which is 3.6 megajoules). This relationship c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Potential
A thermodynamic potential (or more accurately, a thermodynamic potential energy)ISO/IEC 80000-5, Quantities an units, Part 5 - Thermodynamics, item 5-20.4 Helmholtz energy, Helmholtz functionISO/IEC 80000-5, Quantities an units, Part 5 - Thermodynamics, item 5-20.5, Gibbs energy, Gibbs function is a scalar quantity used to represent the thermodynamic state of a system. The concept of thermodynamic potentials was introduced by Pierre Duhem in 1886. Josiah Willard Gibbs in his papers used the term ''fundamental functions''. One main thermodynamic potential that has a physical interpretation is the internal energy . It is the energy of configuration of a given system of conservative forces (that is why it is called potential) and only has meaning with respect to a defined set of references (or data). Expressions for all other thermodynamic energy potentials are derivable via Legendre transforms from an expression for . In thermodynamics, external forces, such as gravity, ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |