|
Ammonium Chloride Route
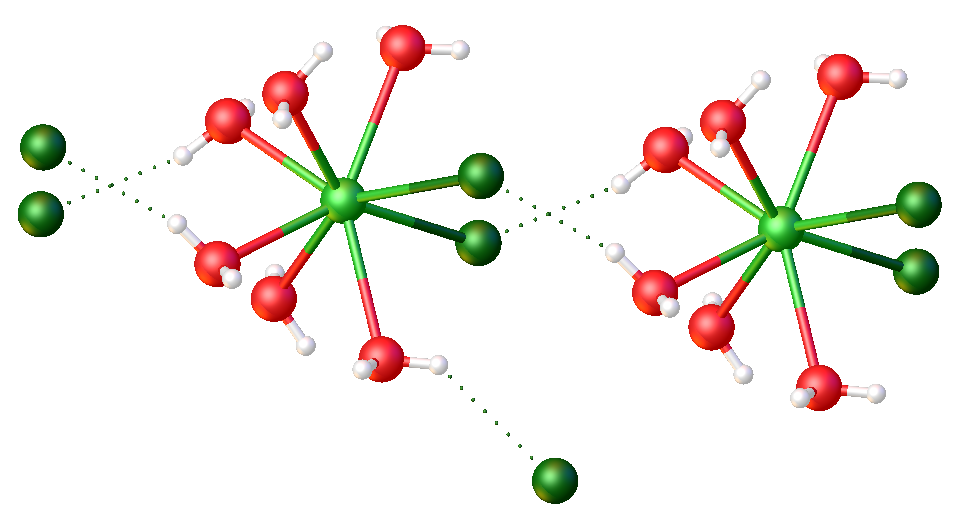
Lanthanide trichlorides are a family of inorganic compound with the formula Ln Cl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates. Properties The anhydrous solids have melting points range from ca. 582 (Tb) - 925 °C (Lu). They are generally pale colored, often white. As coordination polymers, they only dissolve in donor solvents, including water. Preparation The lanthanide oxides and carbonates dissolve in hydrochloric acid to give chloride salt of the hydrated cations: :M2O3 + 6HCl + n H2O → 2 n(H2O)nl3 Industrial routes Anhydrous trichlorides are produced commercially by carbothermic reaction of the oxide: :M2O3 + 3Cl2 + 3C → 2MCl3 + 3CO Ammonium chloride route The ammonium chloride route refers to a general procedure to produce anhydrous lanthanide chlorides. The method has the advantages of being general for the 14 la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |