|
Allicin
Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. The allicin generated is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is part of a defense mechanism against attacks by pests on the garlic plant. Allicin is an oily, slightly yellow liquid that gives garlic its distinctive odor. It is a thioester of sulfenic acid and is also known as allyl thiosulfinate. Its biological activity can be attributed to both its antioxidant activity and its reaction with thiol-containing proteins. Produced in garlic cells, allicin is released upon disruption, producing a potent aroma when garlic is cut or cooked, and is among the chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diallyl Disulfide
Diallyl disulfide (DADS or 4,5-dithia-1,7-octadiene) is an organosulfur compound derived from garlic and a few other genus ''Allium'' plants. Along with diallyl trisulfide and diallyl tetrasulfide, it is one of the principal components of the distilled oil of garlic. It is a yellowish liquid which is insoluble in water and has a strong garlic odor. It is produced during the decomposition of allicin, which is released upon crushing garlic and other plants of the family Alliaceae. Diallyl disulfide has many of the health benefits of garlic, but it is also an allergen causing garlic allergy. Highly diluted, it is used as a flavoring in food. It decomposes in the human body into other compounds such as allyl methyl sulfide. History In 1844, Theodor Wertheim separated by steam distillation a pungent-smelling substance from garlic and named it "allyl sulfur." However, only in 1892 could Friedrich Wilhelm Semmler identify diallyl disulfide as one of the components of distilled garlic oi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plant in the genus ''Allium''. Its close relatives include the onion, shallot, leek, chive, Welsh onion and Chinese onion. It is native to South Asia, Central Asia and northeastern Iran and has long been used as a seasoning worldwide, with a history of several thousand years of human consumption and use. It was known to ancient Egyptians and has been used as both a food flavoring and a traditional medicine. China produces 76% of the world's supply of garlic. Etymology The word ''garlic'' derives from Old English, ''garlēac'', meaning ''gar'' ( spear) and leek, as a 'spear-shaped leek'. Description ''Allium sativum'' is a perennial flowering plant growing from a bulb. It has a tall, erect flowering stem that grows up to . The leaf blade is flat, linear, solid, and approximately wide, with an acute apex. The plant may produce pink to purple flowers from July to September in the Northern Hemisphere. The b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alliinase
In enzymology, an alliin lyase () is an enzyme that catalyzes the chemical reaction :an ''S''-alkyl-L-cysteine ''S''-oxide \rightleftharpoons an alkyl sulfenate + 2-aminoacrylate Hence, this enzyme has one substrate, ''S''-alkyl-L-cysteine ''S''-oxide, and two products, alkyl sulfenate and 2-aminoacrylate. This enzyme belongs to the family of lyases, specifically the class of carbon-sulfur lyases. The systematic name of this enzyme class is ''S''-alkyl-L-cysteine ''S''-oxide alkyl-sulfenate-lyase (2-aminoacrylate-forming). Other names in common use include alliinase, cysteine sulfoxide lyase, alkylcysteine sulfoxide lyase, ''S''-alkylcysteine sulfoxide lyase, L-cysteine sulfoxide lyase, ''S''-alkyl-L-cysteine sulfoxide lyase, and alliin alkyl-sulfenate-lyase. It employs one cofactor, pyridoxal phosphate. Many alliinases contain a novel ''N''-terminal epidermal growth factor-like domain (EGF-like domain). Occurrence These enzymes are found in plants of the genus ''Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfenic Acid
In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula . It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids () and sulfonic acids (), respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide. Properties In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH3SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy ( rotational spectroscopy) to be CH3–S–O–H. Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form thiosulfinates, RS(O)SR, such as allicin from garlic. Through the use of X-ray crystallography, the structure of such stabilized sulfenic acids were shown to be R–S–O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alliin
Alliin is a sulfoxide that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin and other thiosulfinates in garlic are unstable and form a number of other compounds, such as diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DAT), dithiins and ajoene. Garlic powder is not a source of alliin, nor is fresh garlic upon maceration, since the enzymatic conversion to allicin takes place in the order of seconds. Alliin was the first natural product found to have both carbon- and sulfur-centered stereochemistry. Chemical synthesis The first reported synthesis, by Stoll and Seebeck in 1951, begins the alkylation of -cysteine with allyl bromide to form deoxyalliin. Oxidation of this sulfide with hydrogen peroxide gives both diastereomers of -alliin, differing in the orientati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulbutiamine
Sulbutiamine (brand names Arcalion, Enerion) is a synthetic derivative of thiamine (vitamin B1). In France, it is used to treat symptoms of weakness or fatigue. It is also sold as a dietary supplement. Sulbutiamine was discovered in Japan as part of an effort to develop useful thiamine derivatives. Medical use Sulbutiamine is used to treat asthenia (symptoms of fatigue or weakness), though is not clear if it is effective in alleviating tiredness. It is also used to treat thiamine deficiency and poor concentration. Being a potent cholinergic anxiolytic, Sulbutiamine is a popular nootropic, with users reporting enhanced memory, focus and improved mood and motivation. Endurance athletes may use it to try to enhance their performance. Adverse effects Adverse effects in clinical trials have included diarrhea, bladder infections, bronchitis, arthritic pain, back pain, asthma, abdominal pain, insomnia, constipation, gastroenteritis, diffuse pain, sinusitis, headache, kidney pain, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosulfinate
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R (R are organic substituents). Thiolsulfinates are also named as alkanethiosulfinic (or arenethiosulfinic) acid esters. They are the first member of a family of compounds containing an oxidized disulfide bond. Other members of this family include thiosulfonates (R-SO2-S-R), α-disulfoxides (R-S(O)-S(O)-R), sulfinyl sulfones (R-S(O)-SO2-R), and α-disulfones (R-SO2-SO2-R), all of which are known. The thiosulfinate group can occur in cyclic as well as acyclic structures. Occurrence A variety of acyclic and cyclic thiosulfinates are found in plants, or formed when the plants are cut or crushed. A well-known thiosulfinate is allicin, one of the active ingredients formed when garlic is crushed. Allicin was discovered in 1944 by Chester J. Cavallito and coworkers. Thiosulfinates containing various combinations of the methyl, ''n''-propyl, 1-propenyl, 2-propenyl, ''n''-butyl, 1-buten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mustard Plant
The mustard plant is any one of several plant species in the genera ''Brassica'' and '' Sinapis'' in the family Brassicaceae (the mustard family). Mustard seed is used as a spice. Grinding and mixing the seeds with water, vinegar, or other liquids creates the yellow condiment known as prepared mustard. The seeds can also be pressed to make mustard oil, and the edible leaves can be eaten as mustard greens. Many vegetables are cultivated varieties of mustard plants; domestication may have begun 6,000 years ago. History Although some varieties of mustard plants were well-established crops in Hellenistic and Roman times, Zohary and Hopf note, "There are almost no archeological records available for any of these crops." Wild forms of mustard and its relatives, the radish and turnip, can be found over West Asia and Europe, suggesting their domestication took place somewhere in that area. However, Zohary and Hopf conclude: "Suggestions as to the origins of these plants are nec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
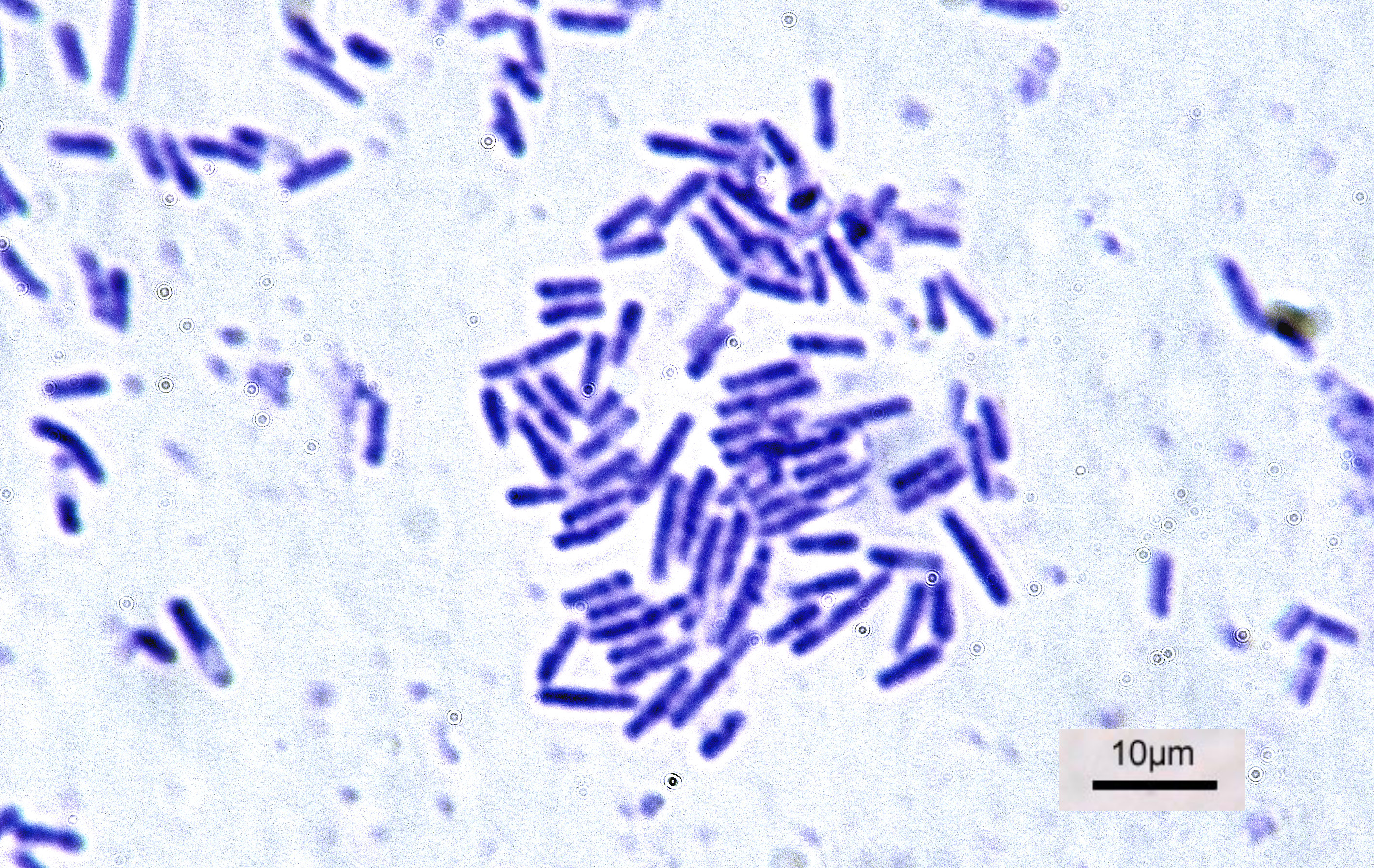
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Isothiocyanate
Allyl isothiocyanate (AITC) is an organosulfur compound (formula CH2CHCH2NCS). This colorless oil is responsible for the pungent taste of mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents. Biosynthesis and biological functions Allyl isothiocyanate can be obtained from the seeds of black mustard (''Brassica nigra'') or brown Indian mustard (''Brassica juncea''). When these mustard seeds are broken, the enzyme myrosinase is released and acts on a glucosinolate known as sinigrin to give allyl isothiocyanate. Allyl isothiocyanate serves the plant as a defense against herbivores; since it is harmful to the plant itself , it is stored in the harmless form of the glucosinolate, separate from the myrosinase enzyme. When an animal chews the plant, the allyl isothiocyanate is released, repelling the animal. Human ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L-glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all anim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |