|
Acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. Synthesis Classic organic reactions exist for the synthesis of acyloins. * The acyloin condensation is a reductive coupling of esters * The benzoin condensation is condensation reaction between aldehydes catalyzed by a nucleophile * Oxidation of carbonyls is possible with molecular oxygen but not selective * Better alternative is oxidation of corresponding silyl enol ethers with ''m''CPBA in the Rubottom oxidation * MoOPH oxidation of carbonyls is a system with molybdenum peroxide, pyridine and hexamethylphosphoramide. Enolate oxidation by sulfonyloxaziridines Enolates can be oxidized by sulfonyloxaziridines. The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine ring. : This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyloin Condensation
Acyloin condensation is a reductive coupling of two carboxylic esters using metallic sodium to yield an α-hydroxyketone, also known as an acyloin. The reaction is most successful when ''R'' is aliphatic and saturated. The reaction is performed in aprotic solvents with a high boiling point, such as benzene and toluene in an oxygen free atmosphere of nitrogen (as even traces of oxygen interfere with the reaction path and reduce the yield). The use of protic solvents results in the Bouveault-Blanc reduction of the separate esters rather than condensation. Depending on ring size and steric properties, but independent from high dilution, the acyloin condensation of diesters favours intramolecular cyclisation over intermolecular polymerisation when diesters are used (see below). To account for such cyclisation, it is suggested that the ends, where ester groups are present, are adsorbed, albeit weakly, at nearby sites on the sodium metal. Thus, the reactive ends are not available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. Synthesis Classic organic reactions exist for the synthesis of acyloins. * The acyloin condensation is a reductive coupling of esters * The benzoin condensation is condensation reaction between aldehydes catalyzed by a nucleophile * Oxidation of carbonyls is possible with molecular oxygen but not selective * Better alternative is oxidation of corresponding silyl enol ethers with ''m''CPBA in the Rubottom oxidation * MoOPH oxidation of carbonyls is a system with molybdenum peroxide, pyridine and hexamethylphosphoramide. Enolate oxidation by sulfonyloxaziridines Enolates can be oxidized by sulfonyloxaziridines. The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine ring. : This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyloin Example Hughes
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. Synthesis Classic organic reactions exist for the synthesis of acyloins. * The acyloin condensation is a reductive coupling of esters * The benzoin condensation is condensation reaction between aldehydes catalyzed by a nucleophile * Oxidation of carbonyls is possible with molecular oxygen but not selective * Better alternative is oxidation of corresponding silyl enol ethers with MCPBA, ''m''CPBA in the Rubottom oxidation * MoOPH oxidation of carbonyls is a system with molybdenum peroxide, pyridine and hexamethylphosphoramide. Enolate oxidation by sulfonyloxaziridines Enolates can be oxidized by oxaziridine, sulfonyloxaziridines. The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoin Condensation
The benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals, and results in formation of an acyloin. In the classic example, benzaldehyde is converted to benzoin. The benzoin condensation was first reported in 1832 by Justus von Liebig and Friedrich Wöhler during their research on bitter almond oil. The catalytic version of the reaction involving cyanide was developed by Nikolay Zinin in the late 1830s. Reaction mechanism The reaction is catalyzed by nucleophiles such as a cyanide or an N-heterocyclic carbene (usually thiazolium salts). The reaction mechanism was proposed in 1903 by A. J. Lapworth. In the first step in this reaction, the cyanide anion (as sodium cyanide) reacts with the aldehyde in a nucleophilic addition. Rearrangement of the intermediate results in polarity reversal of the carbonyl group, which then adds to the second carbonyl group in a second nucleophilic addition. Proto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
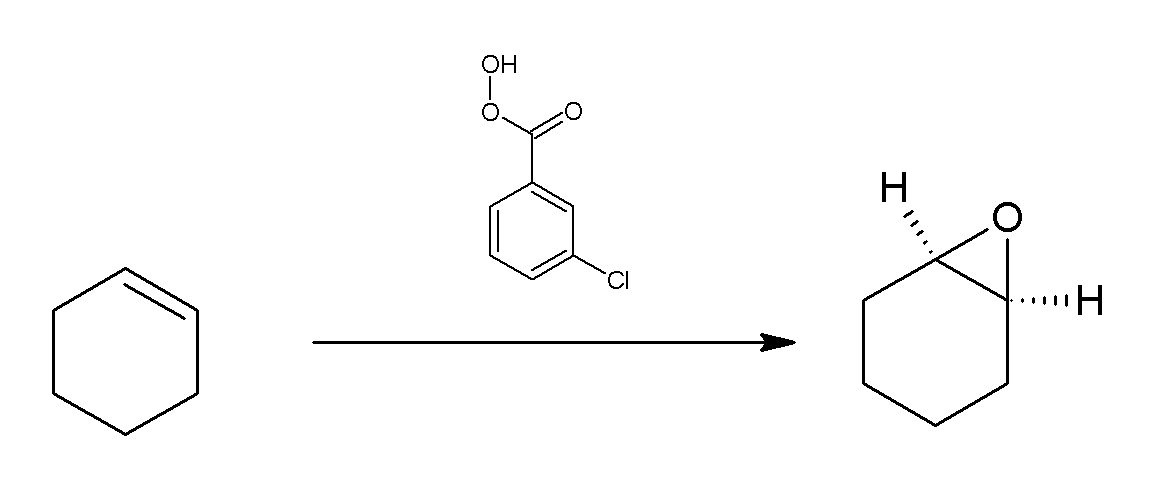
MCPBA
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-Chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so one can extract the acid impurity by careful control of pH. The purified material is reasonably stable against d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Isomer
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules will u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel ('' Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the kapur tree ( ''Dryobalanops'' sp.), a tall timber tree from South East Asia. It also occurs in some other related trees in the laurel family, notably '' Ocotea usambarensis''. Rosemary leaves ('' Rosmarinus officinalis'') contain 0.05 to 0.5% camphor, while camphorweed ('' Heterotheca'') contains some 5%. A major source of camphor in Asia is camphor basil (the parent of African blue basil). Camphor can also be synthetically produced from oil of turpentine. The compound is chiral, existing in two possible enantiomers as shown in the structural diagrams. The structure on the left is the naturally occurring (+)-camphor ((1''R'',4''R'')-bornan-2-one), while its mirror image shown on the right is the (−)-camphor ((1''S'',4''S'')-bornan-2-o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis-trans'' notation does not always correspond to ''E''–''Z'' isomerism, which is an '' absolute'' stereochemical description. In general, ''cis''–''trans'' stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevente ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |