|
Boiling
Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization. There are two main types of boiling: nucleate boiling where small bubbles of vapour form at discrete points, and critical heat flux boiling where the boiling surface is heated above a certain critical temperature and a film of vapour forms on the surface. Transition boiling is an intermediate, unstable form of boiling with elements of both types. The boiling point of water is 100 °C or 212 °F but is lower with the decreased atmospheric pressure found at higher altitudes. Boiling water is used as a method of making it potable by killing Microorganism, microbes and viruses that may be present. The sen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleate Boiling
Nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature (TS) by between . The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling. Nucleate boiling is common in electric kettles and is responsible for the noise that occurs before boiling occurs. It also occurs in water boilers where water is rapidly heated. Mechanism Two different regimes may be distinguished in the nucleate boiling range. When the temperature difference is between approximately above TS, isolated bubbles form at nucleation sites and separate from the surface. This separation induces considerable fluid mixing ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Quality
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Herring, ''General Chemistry'', Prentice-Hall, 8th ed. 2002, p. 483–86. which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas. For example, water has a critical temperature of , which is the highest temperature at which liquid water can exist. In the atmosphere at ordinary temperatures gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently. A vapor may co-exist with a liquid (or a solid). When this is true, the two phases will be in equilibrium, and the gas-partial pressure will be equal to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
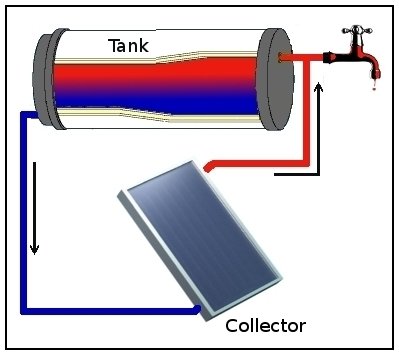
Thermosiphon
Thermosiphon (or thermosyphon) is a method of passive heat exchange, based on natural convection, which circulates a fluid without the necessity of a mechanical pump. Thermosiphoning is used for circulation of liquids and volatile gases in heating and cooling applications such as heat pumps, water heaters, boilers and furnaces. Thermosiphoning also occurs across air temperature gradients such as those utilized in a wood fire chimney or solar chimney. This circulation can either be open-loop, as when the substance in a holding tank is passed in one direction via a heated transfer tube mounted at the bottom of the tank to a distribution point—even one mounted above the originating tank—or it can be a vertical closed-loop circuit with return to the original container. Its purpose is to simplify the transfer of liquid or gas while avoiding the cost and complexity of a conventional pump. Simple thermosiphon Natural convection of the liquid starts when heat transfer to the liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cavitation
Cavitation is a phenomenon in which the static pressure of a liquid reduces to below the liquid's vapour pressure, leading to the formation of small vapor-filled cavities in the liquid. When subjected to higher pressure, these cavities, called "bubbles" or "voids", collapse and can generate shock waves that may damage machinery. These shock waves are strong when they are very close to the imploded bubble, but rapidly weaken as they propagate away from the implosion. Cavitation is a significant cause of wear in some engineering contexts. Collapsing voids that implode near to a metal surface cause cyclic stress through repeated implosion. This results in surface fatigue of the metal causing a type of wear also called "cavitation". The most common examples of this kind of wear are to pump impellers, and bends where a sudden change in the direction of liquid occurs. Cavitation is usually divided into two classes of behavior: inertial (or transient) cavitation and non-inertial c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Bubble
A bubble is a globule of one substance in another, usually gas in a liquid. Due to the Marangoni effect, bubbles may remain intact when they reach the surface of the immersive substance. Common examples Bubbles are seen in many places in everyday life, for example: * As spontaneous nucleation of supersaturated carbon dioxide in soft drinks * As water vapor in boiling water * As air mixed into agitated water, such as below a waterfall * As sea foam * As a soap bubble * As given off in chemical reactions, e.g., baking soda + vinegar * As a gas trapped in glass during its manufacture * As the indicator in a spirit level Physics and chemistry Bubbles form and coalesce into globular shapes because those shapes are at a lower energy state. For the physics and chemistry behind it, see nucleation. Appearance Bubbles are visible because they have a different refractive index (RI) than the surrounding substance. For example, the RI of air is approximately 1.0003 and the RI of water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Boiling
Nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature (TS) by between . The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling. Nucleate boiling is common in electric kettles and is responsible for the noise that occurs before boiling occurs. It also occurs in water boilers where water is rapidly heated. Mechanism Two different regimes may be distinguished in the nucleate boiling range. When the temperature difference is between approximately above TS, isolated bubbles form at nucleation sites and separate from the surface. This separation induces considerable fluid mixing ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Transfer
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, Convection (heat transfer), thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species (mass transfer in the form of advection), either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system. Heat conduction, also called diffusion, is the direct microscopic exchanges of kinetic energy of particles (such as molecules) or quasiparticles (such as lattice waves) through the boundary between two systems. When an object is at a different temperature from another body or its surroundings, heat flows so that the body and the surroundings reach the same temperature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Heat Flux
Critical heat flux (CHF) describes the thermal limit of a phenomenon where a phase change occurs during heating (such as bubbles forming on a metal surface used to heat water), which suddenly decreases the efficiency of heat transfer, thus causing localised overheating of the heating surface. The critical heat flux for ignition is the lowest thermal load per unit area capable of initiating a combustion reaction on a given material (either flame or smoulder ignition). Description When liquid coolant undergoes a change in phase due to the absorption of heat from a heated solid surface, a higher transfer rate occurs. The more efficient heat transfer from the heated surface (in the form of heat of vaporization plus sensible heat) and the motions of the bubbles (bubble-driven turbulence and convection) leads to rapid mixing of the fluid. Therefore, ''boiling heat transfer'' has played an important role in industrial heat transfer processes such as macroscopic heat transfer exchangers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Delay
In thermodynamics, superheating (sometimes referred to as boiling retardation, or boiling delay) is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. This is a so-called ''metastable state'' or ''metastate'', where boiling might occur at any time, induced by external or internal effects.Debenedetti, P.G.Metastable Liquids: Concepts and Principles; Princeton University Press: Princeton, NJ, USA, 1996.Maris, H., Balibar, S. (2000"Negative Pressures and Cavitation in Liquid Helium"Physics Today 53, 29 Superheating is achieved by heating a homogeneous substance in a clean container, free of nucleation sites, while taking care not to disturb the liquid. This may occur by microwaving water in a very smooth container. Disturbing the water may cause an unsafe eruption of hot water and result in burns. Cause Water is said to "boil" when bubbles of water vapor grow without bound, bursting at the surface. For a vapor bubble to e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)