|
Zirconium-97
Naturally occurring zirconium (40Zr) is composed of four stable isotopes (of which one may in the future be found radioactive), and one very long-lived radioisotope (96Zr), a primordial nuclide that decays via double beta decay with an observed half-life of 2.0×1019 years; it can also undergo single beta decay, which is not yet observed, but the theoretically predicted value of t1/2 is 2.4×1020 years. The second most stable radioisotope is 93Zr, which has a half-life of 1.53 million years. Thirty other radioisotopes have been observed. All have half-lives less than a day except for 95Zr (64.02 days), 88Zr (83.4 days), and 89Zr (78.41 hours). The primary decay mode is electron capture for isotopes lighter than 92Zr, and the primary mode for heavier isotopes is beta decay. List of isotopes , - , 78Zr , style="text-align:right" , 40 , style="text-align:right" , 38 , 77.95523(54)# , 50# ms 170 ns, , , 0+ , , , - , rowspan=2, 79Zr , rowspan=2 style= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role. Characteristics Zirconium is a lustrous, greyish-white, soft, ductile, malleable metal that is solid at room temperature, though it is hard and brittle at lesser purities. In powder form, zirconium is highl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay. Nuclear fission of heavy elements was discovered on Monday 19 December 1938, by German chemist Otto Hahn and his assistant Fritz Strassmann in cooperation with Austrian-Swedish physicist Lise Meitner. Hahn understood that a "burst" of the atomic nuclei had occurred. Meitner explained it theoretically in January 1939 along with her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). Like nuclear fusion, for fission to produce energy, the total binding energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Long-lived Fission Product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity it is necessary to isolate them from man and biosphere and to confine them in nuclear waste repositories for geological period of times. Evolution of radioactivity in nuclear waste Nuclear fission produces fission products, as well as actinides from nuclear fuel nuclei that capture neutrons but fail to fission, and activation products from neutron activation of reactor or environmental materials. Short-term The high short-term radioactivity of spent nuclear fuel is primarily from fission products with short half-life. The radioactivity in the fission product mixture is mostly short-lived isotopes such as 131I and 140Ba, after about four months 141Ce, 95Zr/95Nb and 89Sr take the largest share, while after about two or three years the largest share is taken by 144Ce/144Pr, 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has similar ductility to iron. Niobium oxidizes in Earth's atmosphere very slowly, hence its application in jewelry as a hypoallergenic alternative to nickel. Niobium is often found in the minerals pyrochlore and columbite, hence the former name "columbium". Its name comes from Greek mythology: Niobe, daughter of Tantalus, the namesake of tantalum. The name reflects the great similarity between the two elements in their physical and chemical properties, which makes them difficult to distinguish. English chemist Charles Hatchett reported a new element similar to tantalum in 1801 and named it columbium. In 1809, English chemist William Hyde Wollaston wrongly concluded that tantalum and columbium were identical. German chemist Heinrich Rose determin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively. Beta particles with an energy of 0.5 MeV have a range of about one metre in air; the distance is dependent on the particle energy. Beta particles are a type of ionizing radiation and for radiation protection purposes are regarded as being more ionising than gamma rays, but less ionising than alpha particles. The higher the ionising effect, the greater the damage to living tissue, but also the lower the penetrating power of the radiation. Beta decay modes β− decay (electron emission) An unstable atomic nucleus with an excess of neutrons may undergo β− decay, where a neutron is converted into a proton, an electron, and an electron antineutrino (the antip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
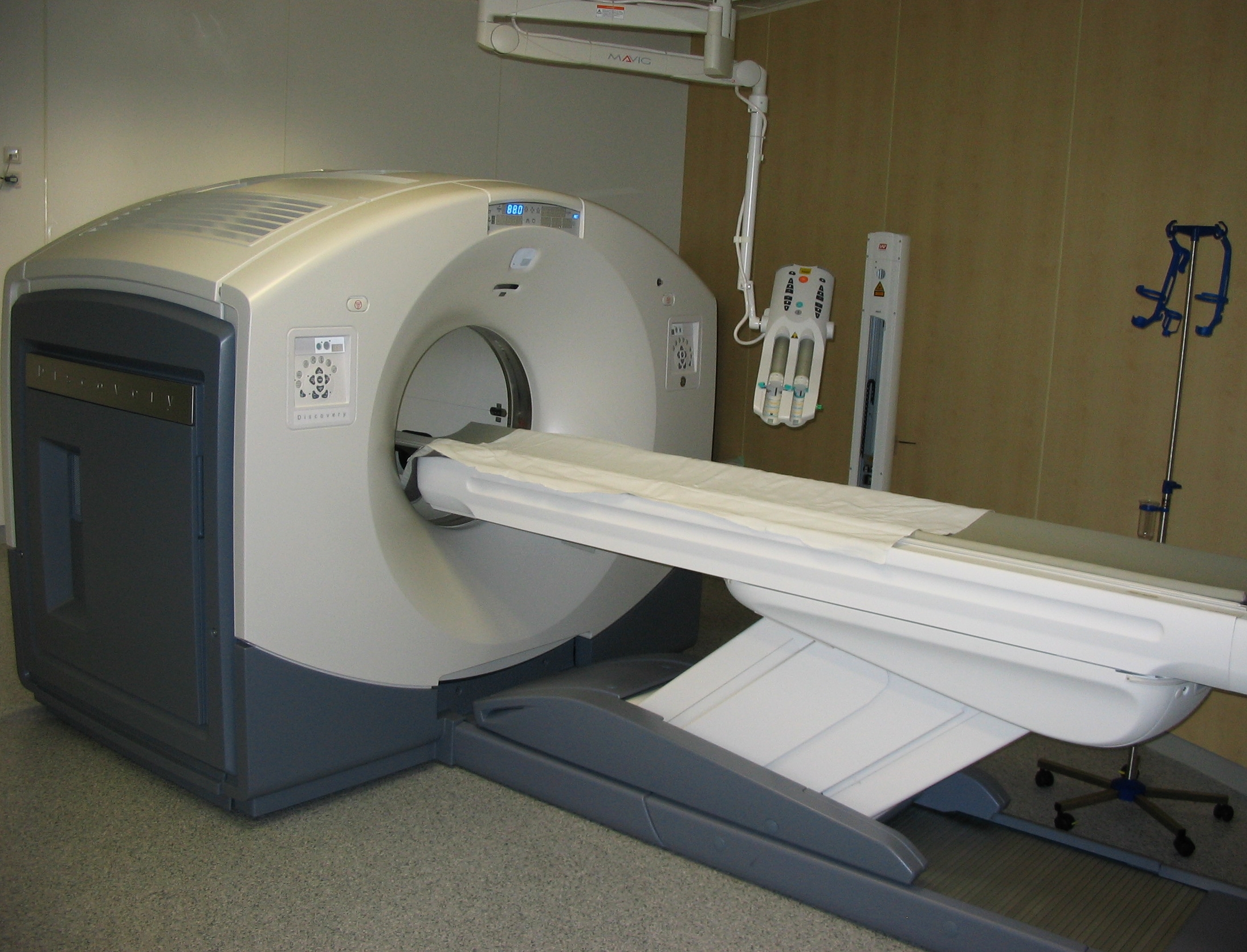
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in Metabolism, metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18F-FDG, -FDG is commonly used to detect cancer, Sodium fluoride#Medical imaging, NaF is widely used for detecting bone formation, and Isotopes of oxygen#Oxygen-15, oxygen-15 is sometimes used to measure blood flow. PET is a common medical imaging, imaging technique, a Scintigraphy#Process, medical scintillography technique used in nuclear medicine. A radiopharmaceutical, radiopharmaceutical — a radioisotope attached to a drug — is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon-135
Xenon-135 (135Xe) is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison (2 million barns; up to 3 million barns under reactor conditions), with a significant effect on nuclear reactor operation. The ultimate yield of xenon-135 from fission is 6.3%, though most of this is from fission-produced tellurium-135 and iodine-135. 135Xe effects on reactor restart In a typical nuclear reactor fueled with uranium-235, the presence of 135Xe as a fission product presents designers and operators with problems due to its large neutron cross section for absorption. Because absorbing neutrons can detrimentally affect a nuclear reactor's ability to increase power, reactors are designed to mitigate this effect; operators are trained to properly anticipate and react to these transients. In fact, during World War II, Enrico Fermi suspected the effect of Xe-135, and followed the advi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross Section (physics)
In physics, the cross section is a measure of the probability that a specific process will take place when some kind of radiant excitation (e.g. a particle beam, sound wave, light, or an X-ray) intersects a localized phenomenon (e.g. a particle or density fluctuation). For example, the Rutherford cross-section is a measure of probability that an alpha particle will be deflected by a given angle during an interaction with an atomic nucleus. Cross section is typically denoted ( sigma) and is expressed in units of area, more specifically in barns. In a way, it can be thought of as the size of the object that the excitation must hit in order for the process to occur, but more exactly, it is a parameter of a stochastic process. In classical physics, this probability often converges to a deterministic proportion of excitation energy involved in the process, so that, for example, with light scattering off of a particle, the cross section specifies the amount of optical power scattere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and quickly deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |