|
Zero Field Splitting
Zero field splitting (ZFS) describes various interactions of the energy levels of a molecule or ion resulting from the presence of more than one unpaired electron. In quantum mechanics, an energy level is called degenerate if it corresponds to two or more different measurable states of a quantum system. In the presence of a magnetic field, the Zeeman effect is well known to split degenerate states. In quantum mechanics terminology, the degeneracy is said to be "lifted" by the presence of the magnetic field. In the presence of more than one unpaired electron, the electrons mutually interact to give rise to two or more energy states. Zero field splitting refers to this lifting of degeneracy even in the absence of a magnetic field. ZFS is responsible for many effects related to the magnetic properties of materials, as manifested in their electron spin resonance spectra and magnetism. The classic case for ZFS is the spin triplet, i.e., the S=1 spin system. In the presence of a magnet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeeman Effect
The Zeeman effect (; ) is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel prize for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden (in the dipole approximation), as governed by the selection rules. Since the distance between the Zeeman sub-levels is a function of magnetic field strength, this effect can be used to measure magnetic field strength, e.g. that of the Sun and other stars or in laboratory plasmas. The Zeeman effect is very important in applications such as nuclear magnetic resonance spectroscopy, electron spin resonance spectroscopy, magnetic resonance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Spin Resonance Spectroscopy
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin Quantum Number
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe the fourth of a set of quantum numbers (the principal quantum number , the azimuthal quantum number , the magnetic quantum number , and the spin quantum number ), which completely describe the quantum state of an electron in an atom. The name comes from a physical spinning of the electron about an axis, as proposed by Uhlenbeck and Goudsmit. The value of is the component of spin angular momentum parallel to a given direction (the –axis), which can be either +1/2 or –1/2 (in units of the reduced Planck constant). However this simplistic picture was quickly realized to be physically impossible because it would require the electrons to rotate faster than the speed of light. It was therefore replaced by a more abstract quantum-mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeeman Splitting
The Zeeman effect (; ) is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel prize for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden (in the dipole approximation), as governed by the selection rules. Since the distance between the Zeeman sub-levels is a function of magnetic field strength, this effect can be used to measure magnetic field strength, e.g. that of the Sun and other stars or in laboratory plasmas. The Zeeman effect is very important in applications such as nuclear magnetic resonance spectroscopy, electron spin resonance spectroscopy, magnetic resonance imagi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamiltonian (quantum Mechanics)
Hamiltonian may refer to: * Hamiltonian mechanics, a function that represents the total energy of a system * Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system ** Dyall Hamiltonian, a modified Hamiltonian with two-electron nature ** Molecular Hamiltonian, the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule * Hamiltonian (control theory), a function used to solve a problem of optimal control for a dynamical system * Hamiltonian path, a path in a graph that visits each vertex exactly once * Hamiltonian group, a non-abelian group the subgroups of which are all normal * Hamiltonian economic program The Hamiltonian economic program was the set of measures that were proposed by American Founding Father and first Secretary of the Treasury Alexander Hamilton in four notable reports and implemented by the US Congress during George Washington's ..., the economic policies advocated by Alexander Hamilton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Dipole–dipole Interaction
Magnetic dipole–dipole interaction, also called dipolar coupling, refers to the direct interaction between two magnetic dipoles. Suppose and are two magnetic dipole moments that are far enough apart that they can be treated as point dipoles in calculating their interaction energy. The potential energy of the interaction is then given by: :: H = -\frac\left 3(\mathbf m_1\cdot\hat\mathbf r)(\mathbf m_2\cdot\hat\mathbf r) - \mathbf m_1\cdot\mathbf m_2\right\mu_0 \frac \mathbf m_1\cdot\mathbf m_2 \delta(\mathbf r) :: where is the magnetic constant, is a unit vector parallel to the line joining the centers of the two dipoles, and , , is the distance between the centers of and . Last term with \delta-function vanishes everywhere but the origin, and is necessary to ensure that \nabla\cdot\mathbf B vanishes everywhere. Alternatively, suppose and are gyromagnetic ratios of two particles with spin quanta and . (Each such quantum is some integral multiple of .) Then: :: H = - ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SQUID
True squid are molluscs with an elongated soft body, large eyes, eight arms, and two tentacles in the superorder Decapodiformes, though many other molluscs within the broader Neocoleoidea are also called squid despite not strictly fitting these criteria. Like all other cephalopods, squid have a distinct head, bilateral symmetry, and a mantle. They are mainly soft-bodied, like octopuses, but have a small internal skeleton in the form of a rod-like gladius or pen, made of chitin. Squid diverged from other cephalopods during the Jurassic and occupy a similar role to teleost fish as open water predators of similar size and behaviour. They play an important role in the open water food web. The two long tentacles are used to grab prey and the eight arms to hold and control it. The beak then cuts the food into suitable size chunks for swallowing. Squid are rapid swimmers, moving by jet propulsion, and largely locate their prey by sight. They are among the most intelligent o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a ve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
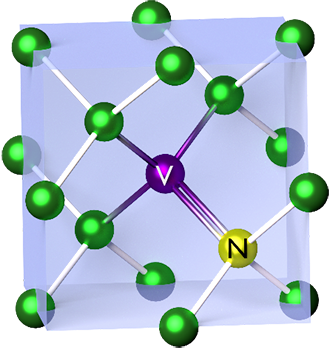
N-V Center
The nitrogen-vacancy center (N-V center or NV center) is one of numerous point defects in diamond. Its most explored and useful property is its photoluminescence, which allows observers to read out its spin-state. The NV center's electron spin, localized at atomic scales, can be manipulated at room temperature by external factors such as magnetic, or electric fields, microwave radiation, or light, resulting in sharp resonances in the intensity of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin–orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual NV center can be used as a basic unit for a quantum computer, a qubit, and used for quantum cryptography. Further potential applications in novel fields of electronics and sensing include spintronics, masers, and quantum sensors. If the charge is not specified the term "NV center" refers to the ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite. Electronic applications of silicon carbide such as light-emitting diodes (LEDs) and detectors in early radios were first demonstrated around 1907. SiC is used in semiconductor electronics devices that operate at high temperatures or high voltages, or both. Natural occurrence Naturally occurring moissanite is found in only minute quantities in cer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)