|
Yellow Fluorescent Protein
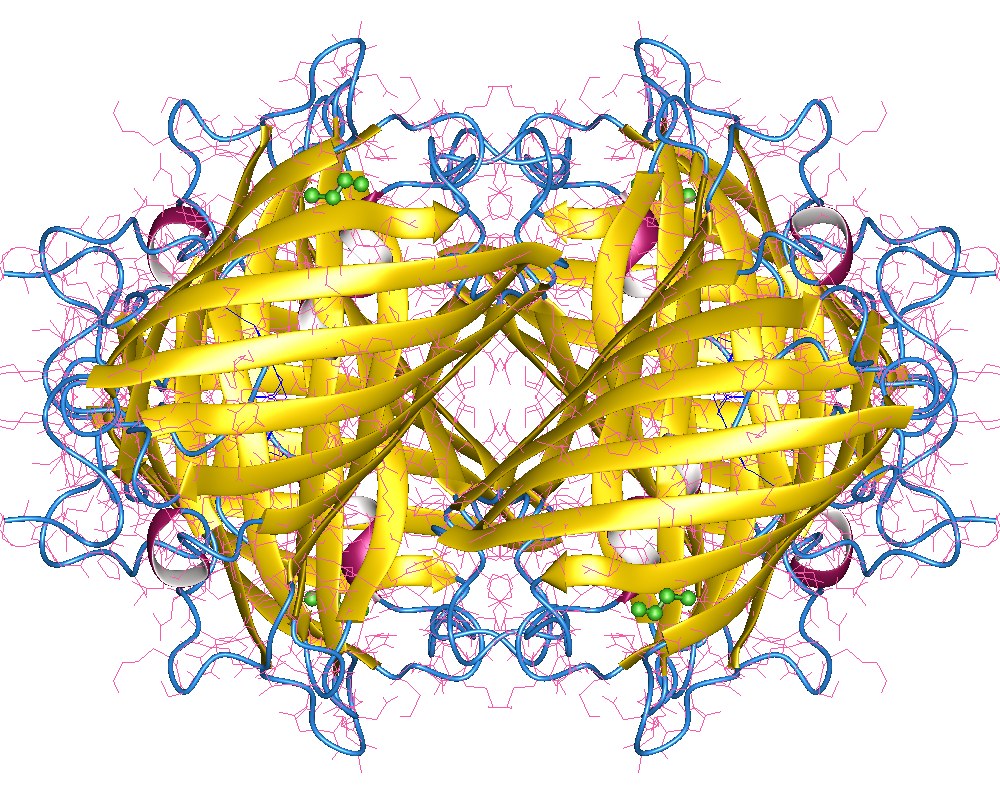
Yellow fluorescent protein (YFP) is a genetic mutant of green fluorescent protein (GFP) originally derived from the jellyfish '' Aequorea victoria''. Its excitation peak is 513 nm and its emission peak is 527 nm. Like the parent GFP, YFP is a useful tool in cell and molecular biology because the excitation and emission peaks of YFP are distinguishable from GFP which allows for the study of multiple processes/proteins within the same experiment. Three improved versions of YFP are Citrine, Venus, and Ypet. They have reduced chloride sensitivity, faster maturation, and increased brightness (defined as the product of the extinction coefficient and quantum yield). Typically, YFP serves as the acceptor for genetically-encoded FRET sensors of which the most likely donor FP is monomeric cyan fluorescent protein (mCFP). The red-shift relative to GFP is caused by a Pi-Pi stacking interaction as a result of the T203Y substitution introduced by mutation, which essentially incre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Fluorescent Protein
The green fluorescent protein (GFP) is a protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. The label ''GFP'' traditionally refers to the protein first isolated from the jellyfish '' Aequorea victoria'' and is sometimes called ''avGFP''. However, GFPs have been found in other organisms including corals, sea anemones, zoanithids, copepods and lancelets. The GFP from ''A. victoria'' has a major excitation peak at a wavelength of 395 nm and a minor one at 475 nm. Its emission peak is at 509 nm, which is in the lower green portion of the visible spectrum. The fluorescence quantum yield (QY) of GFP is 0.79. The GFP from the sea pansy ('' Renilla reniformis'') has a single major excitation peak at 498 nm. GFP makes for an excellent tool in many forms of biology due to its ability to form an internal chromophore without requiring any accessory cofactors, gene products, or enzymes / substrates other than mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jellyfish
Jellyfish and sea jellies are the informal common names given to the medusa-phase of certain gelatinous members of the subphylum Medusozoa, a major part of the phylum Cnidaria. Jellyfish are mainly free-swimming marine animals with umbrella-shaped bells and trailing tentacles, although a few are anchored to the seabed by stalks rather than being mobile. The bell can pulsate to provide propulsion for highly efficient locomotion. The tentacles are armed with stinging cells and may be used to capture prey and defend against predators. Jellyfish have a complex life cycle; the medusa is normally the sexual phase, which produces planula larvae that disperse widely and enter a sedentary polyp phase before reaching sexual maturity. Jellyfish are found all over the world, from surface waters to the deep sea. Scyphozoans (the "true jellyfish") are exclusively marine, but some hydrozoans with a similar appearance live in freshwater. Large, often colorful, jellyfish are common in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aequorea Victoria
''Aequorea victoria'', also sometimes called the crystal jelly, is a bioluminescent hydrozoan jellyfish, or hydromedusa, that is found off the west coast of North America. The species is best known as the source of two proteins involved in bioluminescence, aequorin, a photoprotein, and green fluorescent protein (GFP). Their discoverers, Osamu Shimomura and colleagues, won the 2008 Nobel Prize in Chemistry for their work on GFP. Description Almost entirely transparent and colorless, and sometimes difficult to resolve, ''Aequorea victoria'' possess a highly contractile mouth and manubrium at the center of up to 100 radial canals that extend to the bell margin. The bell margin is surrounded by uneven tentacles, up to 150 of them in fully-grown specimens. The tentacles possess nematocysts that aid in prey capture, although they have no effect on humans. Specimens larger than 3 cm usually possess gonads for sexual reproduction, which run most of the length of the radial cana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, and contains many biomolecules such as proteins, DNA and RNA, as well as many small molecules of nutrients and metabolites.Cell Movements and the Shaping of the Vertebrate Body in Chapter 21 of Molecular Biology of the Cell '' fourth edition, edited by Bruce Alberts (2002) published by Garland Science. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos. It is also common to describe small molecules such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physical structure of biological macromolecules is known as molecular biology. Molecular biology was first described as an approach focused on the underpinnings of biological phenomena - uncovering the structures of biological molecules as well as their interactions, and how these interactions explain observations of classical biology. In 1945 the term molecular biology was used by physicist William Astbury. In 1953 Francis Crick, James Watson, Rosalind Franklin, and colleagues, working at Medical Research Council unit, Cavendish laboratory, Cambridge (now the MRC Laboratory of Molecular Biology), made a double helix model of DNA which changed the entire research scenario. They proposed the DNA structure based on previous research done by R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Absorptivity
Molar may refer to: *Molar (tooth), a kind of tooth found in mammals *Molar (grape), another name for the Spanish wine grape Listan Negro *Molar (unit), a unit of concentration equal to 1 mole per litre *Molar mass * Molar volume *El Molar, Tarragona, a village in the comarca (county) of Priorat, province of Tarragona in the autonomous region of Catalonia, Spain *El Molar, Madrid, a town in the north of the Community of Madrid in the road to Burgos, after San Agustín de Guadalix See also * Moler Moler (previously called Snuff) are a power pop band which formed in 1993 as a three-piece with founding mainstays Helen Cattanach on bass guitar and lead vocals and Julien Poulsen on lead guitar. They featured a changing line-up of drummers and ..., a power-pop band from Australia * Moler (surname) {{disambig, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Yield
The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system. Applications Fluorescence spectroscopy The fluorescence quantum yield is defined as the ratio of the number of photons emitted to the number of photons absorbed.Lakowicz, Joseph R. ''Principles of Fluorescence Spectroscopy'' (Kluwer Academic / Plenum Publishers 1999) p.10. Fluorescence quantum yield is measured on a scale from 0 to 1.0, but is often represented as a percentage. A quantum yield of 1.0 (100%) describes a process where each photon absorbed results in a photon emitted. Substances with the largest quantum yields, such as rhodamines, display the brightest emissions; however, compounds with quantum yields of 0.10 are still considered quite fluorescent. Quantum yield is defined by the fraction of excited state fluorophores that decay through fluorescence: where \Phi_ is the fluorescence quantum yield, k_ is the rate constant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Förster Resonance Energy Transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance. Measurements of FRET efficiency can be used to determine if two fluorophores are within a certain distance of each other. Such measurements are used as a research tool in fields including biology and chemistry. FRET is analogous to near-field communication, in that the radius of interaction is much smaller than the wavelength of light emitted. In the near-field region, the excited chromophore em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stacking (chemistry)
In chemistry, pi stacking (also called π–π stacking) refers to the presumptive attractive, noncovalent pi interactions ( orbital overlap) between the pi bonds of aromatic rings. However this is a misleading description of the phenomena since direct stacking of aromatic rings (the "sandwich interaction") is electrostatically repulsive. What is more commonly observed (see figure to the right) is either a staggered stacking (parallel displaced) or pi-teeing (perpendicular T-shaped) interaction both of which are electrostatic attractive For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare. Pi stacking is repulsive as it places carbon atoms with partial negative charges from one ring on top of other partial negatively charged carbon atoms from the second ring and hydrogen atoms with partial positive charges on top ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromophore
A chromophore is the part of a molecule responsible for its color. The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light. Conjugated pi-bond system chromophores Just like how two adjacent p-orbitals in a molecule will form a pi-bond, three or more adjacent p-orbitals in a molecule can form a conjugated pi-system. In a conjugated pi-system, electrons are able to capture certain photons as the electrons resonate along a certain di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Fluorescent Protein
Red fluorescent protein (RFP) is a fluorophore that fluoresces red-orange when excited. Several variants have been developed using directed mutagenesis. The original was isolated from Discosoma, and named DsRed. Others are now available that fluoresce orange, red, and far-red. RFP is approximately 25.9 kDa. The excitation maximum is 558 nm, and the emission maximum is 583 nm. The first fluorescent protein to be discovered, green fluorescent protein (GFP), has been adapted to identify and develop fluorescent markers in other colors. Variants such as yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) were discovered in Anthozoa. Issues with fluorescent proteins include the length of time between protein synthesis and expression of fluorescence. DsRed has an maturation time of around 24 hours, which can make it unusable for many experiments that take place in a shorter time frame. Additionally, DsRed exists in a tetrameric form, which can affect the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpeg/1200px-Fall_Leaves_(199582361).jpeg)