|
Xenon Dioxide
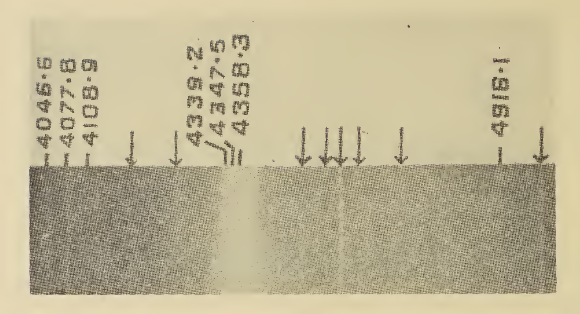
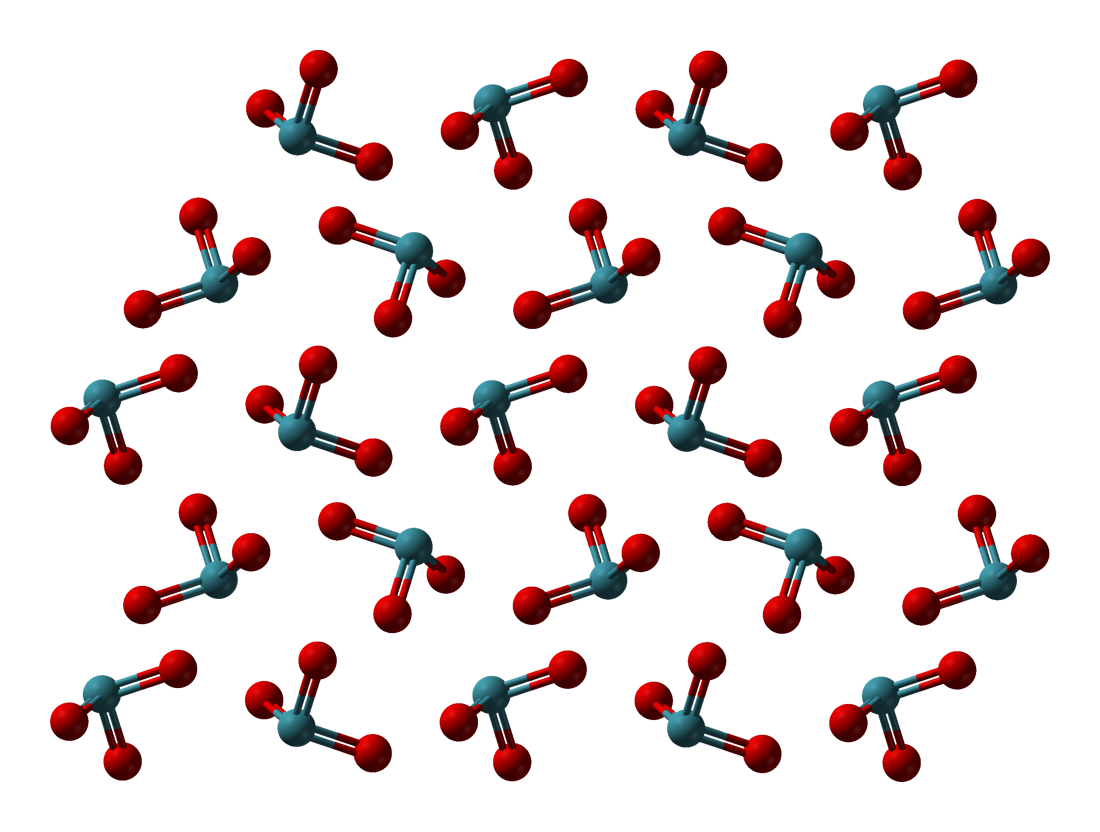
Xenon dioxide, or xenon(IV) oxide, is a compound of xenon and oxygen with formula XeO2 which was synthesized in 2011. It is synthesized at 0 °C by hydrolysis of xenon tetrafluoride in aqueous sulfuric acid: XeF4 + 2H2O -> XeO2 + 4HF Structure has an extended (chain or network) structure in which xenon and oxygen have coordination numbers of four and two respectively. The geometry at xenon is square planar, consistent with VSEPR theory for four ligands and two lone pairs (or AX4E2 in the notation of VSEPR theory). In addition, the existence of an XeO2 molecule was predicted by an ab initio quantum chemistry method several years earlier by Pyykkö and Tamm, but these authors did not consider an extended structure. Properties is a yellow-orange solid. It is an unstable compound, with a half-life of about two minutes, disproportionating into and xenon gas. Its structure and identity was confirmed by cooling it to −78 °C The degree Celsius is the unit of temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bent Molecular Geometry
In chemistry, molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry, also known as angular or V-shaped. Certain atoms, such as oxygen, will almost always set their two (or more) covalent bonds in non-collinear directions due to their electron configuration. Water (H2O) is an example of a bent molecule, as well as its analogues. The bond angle between the two hydrogen atoms is approximately 104.45°. Nonlinear geometry is commonly observed for other triatomic molecules and ions containing only main group elements, prominent examples being nitrogen dioxide (NO2), sulfur dichloride (SCl2), and methylene (CH2). This geometry is almost always consistent with VSEPR theory, which usually explains non-collinearity of atoms with a presence of lone pairs. There are several variants of bending, where the most common is AX2E2 where two covalent bonds and two lone pairs of the central atom (A) form a complete 8-electron shell. They have central angles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon(IV) Compounds
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xenon-135 is pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxides
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated with a laser beam. Electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celsius
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The degree Celsius (symbol: °C) can refer to a specific temperature on the Celsius scale or a unit to indicate a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who developed a similar temperature scale in 1742. Before being renamed in 1948 to honour Anders Celsius, the unit was called ''centigrade'', from the Latin ''centum'', which means 100, and ''gradus'', which means steps. Most major countries use this scale; the other major scale, Fahrenheit, is still used in the United States, some island territories, and Liberia. The Kelvin scale is of use in the sciences, with representing absolute zero. Since 1743 the Celsius scale has been based on 0 °C for the freezing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Trioxide
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas. Chemistry Xenon trioxide is a strong oxidising agent and can oxidise most substances that are at all oxidisable. However, it is slow-acting and this reduces its usefulness. Above 25 °C, xenon trioxide is very prone to violent explosion: :2 XeO3 → 2 Xe + 3 O2 (Δ''H''f = −403 kJ/ mol) When it dissolves in water, an acidic solution of xenic acid is formed: :XeO3(aq) + H2O → H2XeO4 H+ + This solution is stable at room temperature and lacks the explosive properties of xenon trioxide. It oxidises carboxylic acids quantitatively to carbon dioxide and water. Alternatively, it dissolves in alkaline solutions to form ''xenates''. The anion is the predominant specie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. The term is also used more generally to characterize any type of exponential (or, rarely, non-exponential) decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life (in exponential growth) is doubling time. The original term, ''half-life period'', dating to Ernest Rutherford's discovery of the principle in 1907, was shortened to ''half-life'' in the early 1950s. Rutherford applied the principle of a radioactive element's half-life in studies of age determination of rocks by measuring the decay period of radium to lead-206. Half-life is constant over the lifetime of an exponentially decaying quantity, and it is a characteristic unit for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Education In Chemistry
''Education in Chemistry'' (often referred to by its brand 'EiC') is a print and online magazine covering all areas of chemistry education, mainly concentrating on the teaching of chemistry in secondary schools and university, universities. It is published by the Royal Society of Chemistry, which also publishes ''Chemistry Education Research and Practice'', a peer-reviewed academic journal on the same topic. History The feasibility of a "British Journal of Chemistry Education" was first discussed by the Royal Society of Chemistry in late 1962 (a similar journal, the ''Journal of Chemical Education'' had been in existence in the USA since 1924). Its launch was secured by the lobbying of Professor Ronald Sydney Nyholm, Ronald S. Nyholm who became the first Chair of the editorial board. The magazine was launched in 1963 under the editor Dr F. W. Gibbs with the first issue published in January 1964. Gibbs' first editorial, "Scientists and Teachers", set out the aims of the publicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ab Initio Quantum Chemistry Method
''Ab initio'' quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term was first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene. The background is described by Parr. ''Ab initio'' means "from first principles" or "from the beginning", implying that the only inputs into an ''ab initio'' calculation are physical constants. ''Ab initio'' quantum chemistry methods attempt to solve the electronic Schrödinger equation given the positions of the nuclei and the number of electrons in order to yield useful information such as electron densities, energies and other properties of the system. The ability to run these calculations has enabled theoretical chemists to solve a range of problems and their importance is highlighted by the awarding of the Nobel prize to John Pople and Walter Kohn. Accuracy and scaling ''Ab initio'' electronic structure method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Trioxide
Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas. Chemistry Xenon trioxide is a strong oxidising agent and can oxidise most substances that are at all oxidisable. However, it is slow-acting and this reduces its usefulness. Above 25 °C, xenon trioxide is very prone to violent explosion: :2 XeO3 → 2 Xe + 3 O2 (Δ''H''f = −403 kJ/ mol) When it dissolves in water, an acidic solution of xenic acid is formed: :XeO3(aq) + H2O → H2XeO4 H+ + This solution is stable at room temperature and lacks the explosive properties of xenon trioxide. It oxidises carboxylic acids quantitatively to carbon dioxide and water. Alternatively, it dissolves in alkaline solutions to form ''xenates''. The anion is the predominant specie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |