|
X-ray Detector
X-ray detectors are devices used to measure the flux, spatial distribution, spectrum, and/or other properties of X-rays. Detectors can be divided into two major categories: imaging detectors (such as photographic plates and X-ray film (photographic film), now mostly replaced by various digitizing devices like image plates or flat panel detectors) and dose measurement devices (such as ionization chambers, Geiger counters, and dosimeters used to measure the local radiation exposure, dose, and/or dose rate, for example, for verifying that radiation protection equipment and procedures are effective on an ongoing basis). X-ray imaging To obtain an image with any type of image detector the part of the patient to be X-rayed is placed between the X-ray source and the image receptor to produce a shadow of the internal structure of that particular part of the body. X-rays are partially blocked ("attenuated") by dense tissues such as bone, and pass more easily through soft tissues. Are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are barite ( barium sulfate, BaSO4) and witherite ( barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1772, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
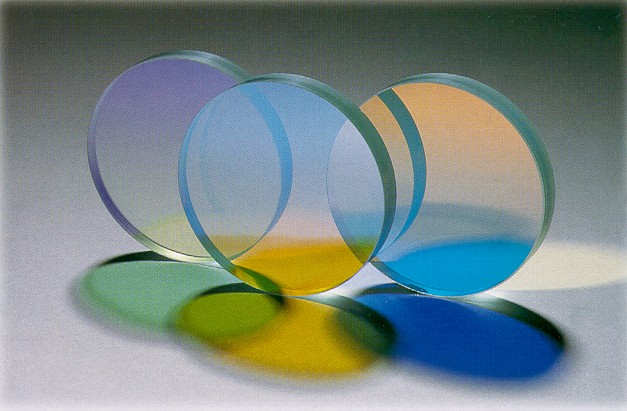
Transparency And Translucency
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) is the physical property of allowing light to pass through the material (with or without scattering of light). It allows light to pass through but the light does not necessarily follow Snell's law on the macroscopic scale; the photons may be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latent Image
A latent image is an invisible image produced by the exposure to light of a photosensitive material such as photographic film. When photographic film is developed, the area that was exposed darkens and forms a visible image. In the early days of photography, the nature of the invisible change in the silver halide crystals of the film's emulsion coating was unknown, so the image was said to be "latent" until the film was treated with photographic developer. In more physical terms, a latent image is a small cluster of metallic silver atoms formed in or on a silver halide crystal due to reduction of interstitial silver ions by photoelectrons (a photolytic silver cluster). If intense exposure continues, such photolytic silver clusters grow to visible sizes. This is called ''printing out'' the image. On the other hand, the formation of a visible image by the action of photographic developer is called ''developing out'' the image. The size of a silver cluster in the latent ima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell parameters in crystals, exhibit a periodic crystal structure, but this is usually imperfect.Ehrhart, P. (1991Properties and interactions of atomic defects in metals and alloys, volume 25 of Landolt-Börnstein, New Series III, chapter 2, p. 88, Springer, Berlin Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological homotopy establishes a mathematical method of characterization. Point defects Point defects are defects that occur only at or around a single lattice point. They are not extended in space in any dimension. Strict limits for how small a point defect is are generally not defined explicitly. However, these defects typically involve at most a few extra or missing atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionised
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons, and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization occur within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Image Resolution
Image resolution is the level of detail of an image. The term applies to digital images, film images, and other types of images. "Higher resolution" means more image detail. Image resolution can be measured in various ways. Resolution quantifies how close lines can be to each other and still be visibly ''resolved''. Resolution units can be tied to physical sizes (e.g. lines per mm, lines per inch), to the overall size of a picture (lines per picture height, also known simply as lines, TV lines, or TVL), or to angular subtense. Instead of single lines, line pairs are often used, composed of a dark line and an adjacent light line; for example, a resolution of 10 lines per millimeter means 5 dark lines alternating with 5 light lines, or 5 line pairs per millimeter (5 LP/mm). Photographic lens are most often quoted in line pairs per millimeter. Types The resolution of digital cameras can be described in many different ways. Pixel count The term ''resolution'' is often considered eq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Bromide
Silver bromide (AgBr), a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for faking the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite (bromyrite). Preparation Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide: :AgNO3(aq) + KBr(aq) → AgBr(s)+ KNO3(aq) Although less convenient, the salt can also be prepared directly from its elements. Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated onto a film or other support. The crystals are formed by precipitation in a controlled environment to prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Halide
A silver halide (or silver salt) is one of the chemical compounds that can form between the Chemical element, element silver (Ag) and one of the halogens. In particular, bromine (Br), chlorine (Cl), iodine (I) and fluorine (F) may each combine with silver to produce silver bromide (AgBr), silver chloride (AgCl), silver iodide (AgI), and four forms of silver fluoride, respectively. As a group, they are often referred to as the silver halides, and are often given the pseudo-chemical notation AgX. Although most silver halides involve silver atoms with oxidation states of +1 (Ag+), silver halides in which the silver atoms have oxidation states of +2 (Ag2+) are known, of which silver(II) fluoride is the only known stable one. Silver halides are light-sensitive chemicals, and are commonly used in photographic film and paper. Applications Light sensitivity Silver halides are used in photographic film and photographic paper, including graphic art film and paper, where silver halide cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorotrast
Thorotrast is a suspension containing particles of the radioactive compound thorium dioxide, ThO2; it was used as a radiocontrast agent in clinical radiography in the 1930s to 1950s. It is no longer used clinically. Thorium compounds produce excellent images because of thorium's high opacity to X-rays (it has a high cross section for absorption). However, thorium is retained in the body, and it is radioactive, emitting harmful alpha radiation as it decays. Because the suspension offered high image quality and had virtually no immediate side-effects compared to the alternatives available at the time, Thorotrast became widely used after its introduction in 1931. António Egas Moniz contributed to its development. About 2 to 10 million patients worldwide have been treated with Thorotrast. However, today it has shown to increase risk of certain cancers, such as cholangiocarcinomas, angiosarcomas and hepatocellular carcinoma, and fibrosis of the liver. Safety Even at the time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |