|
Weston Standard Cell
The Weston standard cell is a wet-chemical cell that produces a highly stable voltage suitable as a laboratory standard for calibration of voltmeters. Invented by Edward Weston in 1893, it was adopted as the International Standard for EMF from 1911 until superseded by the Josephson voltage standard in 1990. Chemistry The anode is an amalgam of cadmium with mercury with a cathode of pure mercury over which a paste of mercurous sulfate and mercury is placed. The electrolyte is a saturated solution of cadmium sulfate, and the depolarizer is a paste of mercurous sulfate. As shown in the illustration, the cell is set up in an H-shaped glass vessel with the cadmium amalgam in one leg and the pure mercury in the other. Electrical connections to the cadmium amalgam and the mercury are made by platinum wires fused through the lower ends of the legs. ;Anode reaction: Cd(s) ŌåÆ Cd2+(aq) + 2eŌłÆ ;Cathode reaction: (Hg+)2(s) + 2eŌłÆ ŌåÆ 2Hg(l) + (aq) Reference cells must be app ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver and was formerly named hydrargyrum ( ) from the Greek words, ''hydor'' (water) and ''argyros'' (silver). A heavy, silvery d-block A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ... element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by Mill (grinding), grinding natural cinnabar or synthetic mercuric sulfide. Mercury is used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Battery Types ...
This list is a summary of notable electric battery types composed of one or more electrochemical cells. Three lists are provided in the table. The primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. The third list is a list of battery applications. Battery cell types See also * Baghdad Battery * Battery nomenclature * Carnot battery * Comparison of commercial battery types * History of the battery * List of battery sizes * List of energy densities * ''Search for the Super Battery'' (2017 PBS film) * Fuel cell References {{Battery sizes * Battery Battery most often refers to: * Electric battery, a device that provides electrical power * Battery (crime), a crime involving unlawful physical contact Battery may also refer to: Energy source *Automotive battery, a device to provide power t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clark Cell
The Clark cell, invented by English engineer Josiah Latimer Clark in 1873, is a wet-chemical cell (colloquially: ''battery'') that produces a highly stable voltage. In 1893, the output of the Clark cell at 15 ┬░C was defined by the International Electrical Congress as 1.434 volts, and this definition became law in the United States in 1894. This definition was later supplanted by one based on the Weston cell. Chemistry Clark cells use a zinc, or zinc amalgam, anode and a mercury cathode in a saturated aqueous solution of zinc sulfate, with a paste of mercurous sulfate as depolarizer. Construction Original cell Clark's original cell was set up in a glass jar in a similar way to a gravity Daniell cell. The copper cathode was replaced by a pool of mercury at the bottom of the jar. Above this was the mercurous sulfate paste and, above that, the zinc sulfate solution. A short zinc rod dipped into the zinc sulfate solution. The zinc rod was supported by a cork with two holes Ō ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature Coefficient
A temperature coefficient describes the relative change of a physical property that is associated with a given change in temperature. For a property ''R'' that changes when the temperature changes by ''dT'', the temperature coefficient ╬▒ is defined by the following equation: :\frac = \alpha\,dT Here ╬▒ has the dimension of an inverse temperature and can be expressed e.g. in 1/K or KŌłÆ1. If the temperature coefficient itself does not vary too much with temperature and \alpha\Delta T \ll 1, a linear approximation will be useful in estimating the value ''R'' of a property at a temperature ''T'', given its value ''R''0 at a reference temperature ''T''0: :R(T) = R(T_0)(1 + \alpha\Delta T), where ╬ö''T'' is the difference between ''T'' and ''T''0. For strongly temperature-dependent ╬▒, this approximation is only useful for small temperature differences ╬ö''T''. Temperature coefficients are specified for various applications, including electric and magnetic properties of materials a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 ╬╝g/kg. It occurs in some nickel and copper ores along with some native deposits, mostly in South Africa, which accounts for ~80% of the world production. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable and is a major precious metal commodity. Platinum is one of the least reactive metals. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
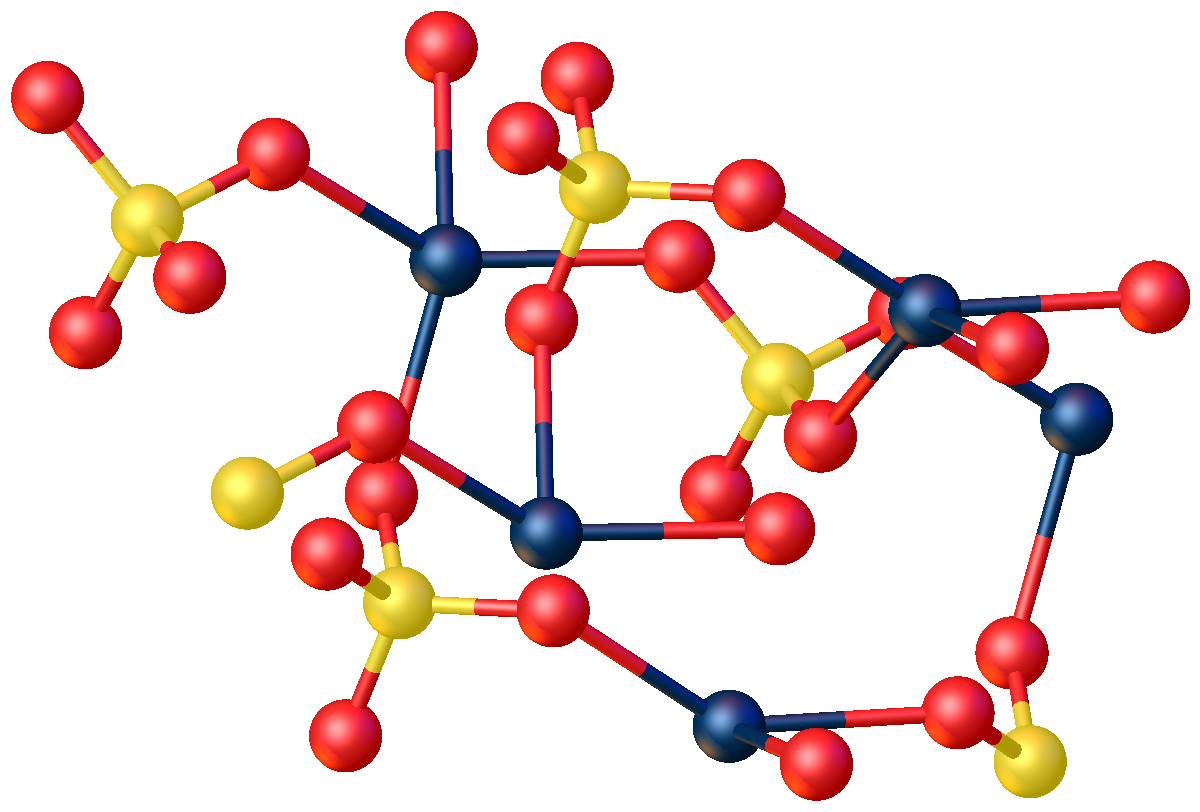
Mercurous Sulfate
Mercury(I) sulfate, commonly called mercurous sulphate ( UK) or mercurous sulfate ( US) is the chemical compound Hg2SO4. Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin. Structure In the crystal, mercurous sulfate is made up of Hg22+ center with an Hg-Hg distance of about 2.50 ├ģ. The SO42ŌłÆ anions form both long and short Hg-O bonds ranging from 2.23 to 2.93 ├ģ. Focusing on the shorter Hg-O bonds, the Hg ŌĆō Hg ŌĆō O bond angle is 165┬░┬▒1┬░. Preparation One way to prepare mercury(I) sulfate is to mix the acidic solution of mercury(I) nitrate with 1 to 6 sulfuric acid solution:, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depolarizer
A depolarizer or depolariser, in electrochemistry, according to an IUPAC definition, is a synonym of electroactive substance, i.e., a substance which changes its oxidation state, or partakes in a formation or breaking of chemical bonds, in a charge-transfer step of an electrochemical reaction. In the battery industry, the term "depolarizer" has been used to denote a substance used in a primary cell to prevent buildup of hydrogen gas bubbles."McGraw-Hill Dictionary of Scientific & Technical Terms", McGraw-Hill, Inc., 2003. A battery depolarizer takes up electrons during discharge of the cell; therefore, it is always an oxidizing agent. The term "depolarizer" can be considered as outdated or misleading, since it is based on the concept of " polarization" which is hardly realistic in many cases. Polarization Under certain conditions for some electrochemical cells, especially if they use an aqueous electrolyte, hydrogen ions can be converted into hydrogen atoms and H2 molecules. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Sulfate
Cadmium sulfate is the name of a series of related inorganic compounds with the formula CdSO4┬ĘH2O. The most common form is the monohydrate CdSO4┬ĘH2O, but two other forms are known CdSO4┬ĘH2O and the anhydrous salt (CdSO4). All salts are colourless and highly soluble in water. Structure, preparation, and occurrence X-ray crystallography shows that CdSO4┬ĘH2O is a typical coordination polymer. Each Cd2+ center has octahedral coordination geometry, being surrounded by four oxygen centers provided by four sulfate ligands and two oxygen centers from the bridging water ligands. Cadmium sulfate hydrate can be prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid: : CdO + H2SO4 ŌåÆ CdSO4 + H2O : Cd + H2SO4 ŌåÆ CdSO4 + H2 The anhydrous material can be prepared using sodium persulfate: : Cd + Na2S2O8 ŌåÆ CdSO4 + Na2SO4 Cadmium sulfates occur as the following rare minerals drobecite (CdSO4┬Ę4H2O), voudourisite (monohydrate), and lazaridisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Solution
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "GasŌĆöGas Equilibria". ''Journal of Chemical Physics'', vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic ''cathode current departs'' also means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside of the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity with respect to the anode can be positive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |