|
Wieland-Gumlich Aldehyde
The so-called Wieland-Gumlich aldehyde (6) is an indoline derived by chemical degradation from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 4 steps from strychnine (1) by Walter Gumlich and Koozoo Kaziro working in the laboratory of Heinrich Wieland. This degradation study was part of an attempt to elucidate the chemical structure of strychnine. This degradation takes place through conversion of strychnine to the oxime 2 using amyl nitrite, Beckmann fragmentation of 2 to the carbamic acid 3 by use of thionyl chloride, decarboxylation of 3 to nitrile 4, and nucleophilic displacement of cyanide by barium hydroxide to give hemiacetal 5, which is in equilibrium with the Wieland-Gumlich aldehyde (6). : The Wieland-Gumlich aldehyde reverts to strychnine in a single reaction using malonic acid, acetic anhydride and sodium acetate in acetic acid. The Wieland-Gumlich aldehyde has been used in the industrial synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indoline
Indoline is an aromatic heterocyclic organic compound with the chemical formula C8H9N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound is based on the indole structure, but the 2-3 bond is saturated Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds ** Saturated and unsaturated compounds **Degree of unsaturation ** Saturated fat or fatty ac .... By oxidation/dehydrogenation it can be converted to indoles. Indoline was used to make Indocaine. References {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemiketals. Most sugars are hemiacetals. Nomenclature According to the IUPAC definition, in R1R2C(OH)OR R1 and R2 may or may not be a hydrogen. In a hemiketal, neither R-group can be a hydrogen. Hemiketals are regarded as hemiacetals where none of the R-groups are H, and are therefore a subclass of the hemiacetals. The Greek prefix ''hèmi'' means half, refers to the fact that a single alcohol has been added to the carbonyl group, in contrast to acetals or ketals, which are formed when a second alkoxy group has been added to the structure. Cyclic hemiacetals and hemiketals are sometimes called lactols.IUPAC Gold Boolactols/ref> They often form readily, especially when they are 5- and 6-membered rings. In this case an intramolecular OH group r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuasca brews. Many synthetic tryptamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimerization (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcuronium Chloride
Alcuronium chloride (formerly marketed as Alloferin) is a neuromuscular blocking (NMB) agent, alternatively referred to as a skeletal muscle relaxant. It is a semi-synthetic substance prepared from C-toxiferine I, a ''bis''-quaternary alkaloid obtained from ''Strychnos toxifera''. C-toxiferine I itself has been tested for its pharmacological action and noted to be a very long acting neuromuscular blocking agent For a formal definition of the durations of actions associated with NMB agents, see page for gantacurium. The replacement of both the ''N''-methyl groups with ''N-allyl'' moieties yielded ''N,N''-diallyl-''bis''-nortoxiferine, now recognized as alcuronium. Inclusion of the allylic functions presented an enhanced potential area of biotransformation, and thus alcuronium is observed to have a much shorter duration of neuromuscular blocking action than its parent C-toxiferine I. It also has a more rapid onset of action, and is ~1.5 times as potent as tubocurarine. The pharmac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses. Applications Biotechnological Sodium acetate is used as the carbon source for culturing bacteria. Sodium acetate is also useful for increasing yields of DNA isolation by ethanol precipitation. Industrial Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams and also as a photoresist while using aniline dyes. It is also a pickling agent in chrome tanning and helps to impede vulcanization of chloroprene in synthetic rubber production. In processing cotton for disposable cotton pads, sodium acetate is used to eliminate the buildup of static electricity. Concrete longevity Sodium acetate is used to mitigate water damage to concrete by acting as a concrete sealant, while also being environmentally benign and cheaper than the commonly used epoxy alternative for sealing concrete against water permeation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air. Structure and properties Acetic anhydride, like most acid anhydrides, is a flexible molecule with a nonplanar structure. The pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low. Like most acid anhydrides, the carbonyl carbon atom of acetic anhydride has electrophilic character, as the leaving group is carboxylate. The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as the asymmetric geometry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonic Acid
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (''malon'') meaning 'apple'. History Malonic acid is a naturally occurring substance found in many fruits and vegetables. There is a suggestion that citrus fruits produced in organic farming contain higher levels of malonic acid than fruits produced in conventional agriculture. Malonic acid was first prepared in 1858 by the French chemist Victor Dessaignes via the oxidation of malic acid. Structure and preparation The structure has been determined by X-ray crystallography and extensive property data including for condensed phase thermochemistry are available from the National Institute of Standards and Technology. A classical preparation of malonic acid starts from chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
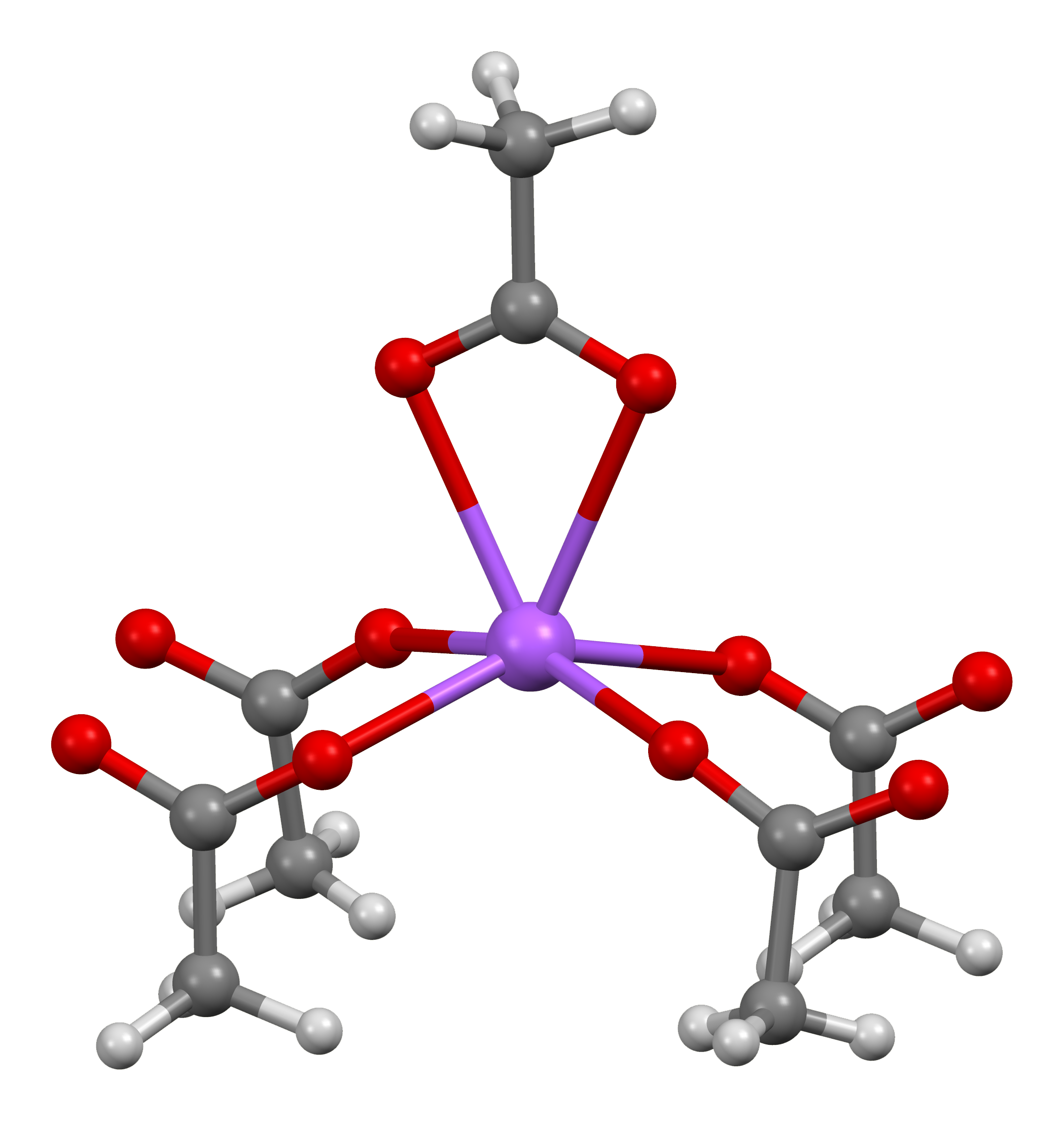
Barium Hydroxide
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (''x'' = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form. Preparation and structure Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water: :BaO + H2O → Ba(OH)2 It crystallises as the octahydrate, which converts to the monohydrate upon heating in air. At 100 °C in a vacuum, the monohydrate will yield BaO and water. The monohydrate adopts a layered structure (see picture above). The Ba2+ centers adopt a square anti-prismatic geometry. Each Ba2+ center is bound by two water ligands and six hydroxide ligands, which are respectively doubly and triply bridging to neighboring Ba2+ centre sites. In the octahydrate, the individual Ba2+ centers are again eight coordinate but do not share ligands. Uses Industrially, barium hydroxide is used as the precursor to other bar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |