|
Vinyl Bromide
Vinyl bromide is a simple vinyl halide. It is a colorless liquid. It is produced from ethylene dibromide. It is mainly used as a comonomer to confer fire retardant properties to acrylate polymers. Reactions and applications It reacts with magnesium to give the corresponding Grignard reagent. Safety precautions Vinyl bromide is listed in List of IARC Group 2A carcinogens as a suspected human carcinogen. See also * Vinyl chloride * Allyl bromide Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and p ... * Bromoethane References External links * *MSDS at Oxford University [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
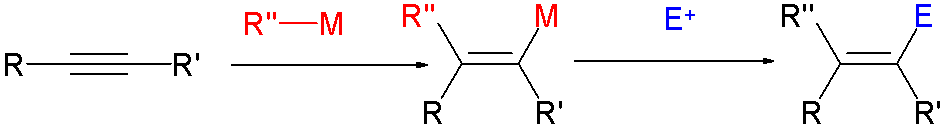
Vinyl Halide
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl halides. From the perspective of applications, the dominant member of this class of compounds is vinyl chloride, which is produced on the scale of millions of tons per year as a precursor to polyvinyl chloride. Polyvinyl fluoride is another commercial product. Related compounds include 1,1-Dichloroethene, vinylidene chloride and vinylidene fluoride. Synthesis Vinyl chloride is produced by dehydrochlorination of 1,2-dichloroethane. Due to their high utility, many approaches to vinyl halides have been developed, such as: * reactions of vinyl organometallic species with halogens * Takai olefination * Stork-Zhao olefination - a modification of the Wittig reaction * Olefin metathesis Reactions Vinyl bromide and related alkenyl halid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Dibromide
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint sweet odor, detectable at 10 ppm, is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive. Preparation and use It is produced by the reaction of ethylene gas with bromine, in a classic halogen addition reaction: :CH=CH + Br → BrCH–CHBr Historically, 1,2-dibromoethane was used as a component in anti-knock additives in leaded fuels. It reacts with lead residues to generate volatile lead bromides, thereby preventing fouling of the engine with lead deposits. Pesticide It has been used as a pesticide in soil and on various crops. The applications were initiated after the forced retirement of 1,2-dibromo-3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comonomer
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furthermore, in some cases, the comonomers are statistically incorporated within the polymer chain, whereas in other cases, they aggregate. The distribution of comonomers is referred to as the " blockiness" of a copolymer. 1-Octene, 1-hexene, and 1-butene are used comonomers in the manufacture of polyethylenes. The advantages to such copolymers has led to a focus on catalysts that facilitate the incorporation of these comonomers, e.g., constrained geometry complexes. Comonomers are often employed to improve the plastification of polymeric materials, i.e. the flexibility of the polymer. Unlike traditional plasticizers, comonomers are not leachable. In other cases, comonomers are used to introduce crosslinking. Divinylbenzene Divinylbenz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Retardant
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are also available as coatings or sprays to be applied to an object. Fire retardants are commonly used in fire fighting, where they may be applied aerially or from the ground. Principles of operation In general, fire retardants reduce the flammability of materials by either blocking the fire physically or by initiating a chemical reaction that stops the fire. Physical action There are several ways in which the combustion process can be retarded by physical action: * By cooling: Some chemical reactions actually cool the material d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylate Polymer
An acrylate polymer (also known as acrylic or polyacrylate) is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity. Acrylate polymer is commonly used in cosmetics, such as nail polish, as an adhesive. History The first synthesis of acrylic polymer was reported by G.W.A Kahlbaum in 1880. Acrylic elastomers Acrylic elastomer is a general term for a type of synthetic rubber whose primary component is acrylic acid alkylester ( ethyl or butyl ester). Acrylic elastomer possesses characteristics of heat and oil resistance, with the ability to withstand temperatures of 170–180 °C. It is used primarily for producing oil seals and packaging related to automobiles. Acrylic elastomer can generally be characterized as one of two types. "Old" types include ACM (copolymer of acrylic acid ester and 2-chloroethyl vinyl ether) containing chlorine and ANM (copolymer of acrylic acid este ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of IARC Group 2A Carcinogens
The agents in this list have been classified in group 2A (probable carcinogens) by the International Agency for Research on Cancer (IARC). The term "agent" encompasses both substances and exposure circumstances that pose a risk. This designation is applied when there is ''limited evidence'' of carcinogenicity in humans as well as ''sufficient evidence'' of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is ''inadequate evidence'' of carcinogenicity in humans along with ''sufficient evidence'' of carcinogenicity in experimental animals and ''strong evidence'' that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of ''limited evidence'' of carcinogenicity in humans. Agents Substances *Acrylamide *Adriamycin * Androgenic (anabolic) steroids *Azacitidine * BCNU (Bischloroethyl nitrosourea) *Captafol *Chloral *Chloral hydrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious. Carcinogens, as mentioned, are agents in the environment capable of contributing to cancer growth. Carcinogens can be categorized into two different types: activation-dependent and activation-independent, and each nature impacts their level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). About 13 million metric tonne are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM. Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Bromide
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and persistent smell, however, commercial samples are yellow or brown. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use. Preparation Allyl bromide is produced commercially from allyl alcohol and hydrobromic acid: :CH2=CHCH2OH + HBr → CH2=CHCH2Br + H2O It can also be prepared by the halogen-exchange reaction between allyl chloride and hydrobromic acid or by the allylic bromination of propene. Reactions and uses Electrophilic properties Allyl bromide is an electrophilic alkylating agent. It reacts with nucleophiles, such as amines, carbanions, alkoxides, etc., to introduce the allyl group: :CH2=CHCH2Br + Nu− → CH2=CHCH2Nu + Br− (Nu− is a nucleophile) It is used in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor. Preparation The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene: :H2C=CH2 + HBr → H3C-CH2Br Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated ''in situ''. Uses In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon. In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters, carbanions to ethylated derivatives, thiour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organobromides
Organobromine compounds, also called organobromides, are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application of synthetic organobromine compounds is the use of polybrominated diphenyl ethers as fire-retardants, and in fact fire-retardant manufacture is currently the major industrial use of the element bromine. A variety of minor organobromine compounds are found in nature, but none are biosynthesized or required by mammals. Organobromine compounds have fallen under increased scrutiny for their environmental impact. General properties Most organobromine compounds, like most organohalide compounds, are relatively nonpolar. Bromine is more electronegative than carbon (2.9 vs 2.5). Consequently, the carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating agents. Carbon–halogen bond strengths, or bond dissociation energies are of 115, 83.7, 72.1, and 57.6 kc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |