|
VigiBase
VigiBase is a World Health Organization's (WHO) global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO's international drug monitoring programme. It is the single largest drug safety data repository in the world. Since 1978, the Uppsala Monitoring Centre (UMC; established in Uppsala, Sweden) on behalf of WHO, have been maintaining VigiBase. Vigibase is used to obtain the information about a safety profile of a medicinal product. These data are used by pharmaceutical industries, academic institutions and regulatory authorities for statistical signal detection, updating periodic reports, ICSR comparisons with company databases and studying the reporting patterns. The data (pre-dominantly post-marketing serious and non-serious cases) is collected from each of its 110 member states which currently comprises to over 10 million ICSRs (October 2014). About a hundred thousand ICSRs are added each year. Histori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uppsala Monitoring Centre
Uppsala Monitoring Centre (UMC), located in Uppsala, Sweden, is the field name for the World Health Organization Collaborating Centre for International Drug Monitoring. UMC works by collecting, assessing and communicating information from member countries' national pharmacovigilance centres in regard to the benefits, harm, effectiveness and risks of drugs. Background Since 1978, responsibility for managing the WHO Programme for International Drug Monitoring has been carried by UMC. In the early years the staff consisted of just three pharmacists based at the Swedish Medical Products Agency (Läkemedelsverket); currently over 100 staff work in central Uppsala. The founding chairman and acting Director was Professor Åke Liljestrand. From 1990 to 2009 the Director was Professor Ralph Edwards. Since September 2009 Dr. Marie Lindquist is the Director. The Chief Medical Officer is Dr. Pia Caduff and the Head of Research is Dr. Niklas Norén. The work of the UMC is: * To co-ordinate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacovigilance
Term Given By Tushar Sharma (UPES Batch 2025) Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of health". Headquartered in Geneva, Switzerland, it has six regional offices and 150 field offices worldwide. The WHO was established on 7 April 1948. The first meeting of the World Health Assembly (WHA), the agency's governing body, took place on 24 July of that year. The WHO incorporated the assets, personnel, and duties of the League of Nations' Health Organization and the , including the International Classification of Diseases (ICD). Its work began in earnest in 1951 after a significant infusion of financial and technical resources. The WHO's mandate seeks and includes: working worldwide to promote health, keeping the world safe, and serve the vulnerable. It advocates that a billion more people should have: universal health care coverag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceuticals Policy
Pharmaceutical policy is a branch of health policy that deals with the development, provision and use of medications within a health care system. It embraces drugs (both brand name and generic), biologics (products derived from living sources, as opposed to chemical compositions), vaccines and natural health products. Funding of research in the life sciences In many countries, an agency of the national government (in the U.S. the NIH, in the U.K. the MRC, and in India the DST) funds university researchers to study the causes of disease, which in some cases leads to the development of discoveries which can be transferred to pharmaceutical companies and biotechnology companies as a basis for drug development. By setting its budget, its research priorities and making decisions about which researchers to fund, there can be a significant impact on the rate of new drug development and on the disease areas in which new drugs are developed. For example, a major investment by the NIH into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacological Classification Systems
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism (sometimes the word ''pharmacon'' is used as a term to encompass these endogenous and exogenous bioactive species). More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals. The field encompasses drug composition and properties,functions,sources,synthesis and drug design, molecular and cellular mechanisms, organ/systems mechanisms, signal transduction/cellular communication, molecular diagnostics, interactions, chemical biology, therapy, and medical applications and antipathogenic capabilities. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WHOART
The WHO Adverse Reactions Terminology (WHOART) is a dictionary meant to serve as a basis for rational coding of adverse reaction terms. The system is maintained by the Uppsala Monitoring Centre (UMC), the World Health Organization Collaborating Centre for International Drug Monitoring. The system is no longer actively maintained. Structure * 32 System-organ classesbody organ groups * 180 High level terms for grouping Preferred terms * 2085 Preferred terms principal terms for describing adverse reactions * 3445 Included terms synonyms to Preferred terms See also *Pharmacovigilance *COSTART *MedDRA *Adverse event An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can ther ... References WHO Adverse Reactions Terminology Medical classification Pharmacological classification systems {{treatm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MedDRA
A subscription-based product of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology dictionary-thesaurus used by regulatory authorities and the biopharmaceutical industry during the regulatory process, from pre-marketing (clinical research phase 0 to phase 3) to post-marketing activities (pharmacovigilance or clinical research phase 4), and for safety information data entry, retrieval, evaluation, and presentation. Also, it is the adverse event classification dictionary. The first version of MedDRA was released in 1999 in English and Japanese. MedDRA is now translated into Chinese, Czech, Dutch, French, German, Hungarian, Italian, Korean, Portuguese, Brazilian Portuguese, Russian, and Spanish. In MedDRA version 25.0, Swedish and Latvian translations were also added. In many countries/regions the use of MedD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
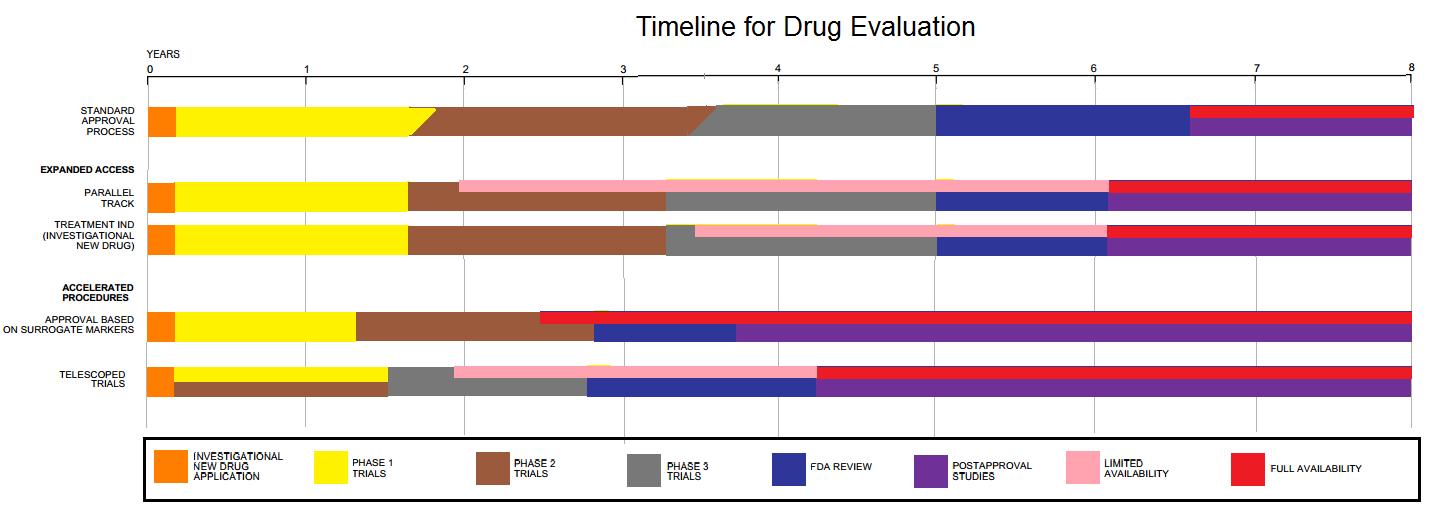
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yellow Card Scheme
The Yellow Card Scheme is the United Kingdom's system for collecting information on suspected adverse drug reactions (ADRs) to medicines. The scheme allows the safety of the medicines and vaccines that are on the market to be monitored. History The scheme was founded in 1964 after the thalidomide disaster, and was developed by Bill Inman. It is run by the Medicines and Healthcare products Regulatory Agency (MHRA) and the Commission on Human Medicines. It was extended to hospital pharmacists in 1997, and to community pharmacists in 1999. The Yellow Card Centre Scotland is a joint venture between MHRA and the Scottish Government. Scope Suspected adverse reactions are collected on all licensed medicines and vaccines, whether issued on prescription or bought over the counter from a pharmacist or supermarket. The scheme also includes all herbal preparations and unlicensed medicines. Adverse reactions can be reported by anyone; this is usually done by healthcare professionals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EudraVigilance
EudraVigilance (European Union Drug Regulating Authorities Pharmacovigilance) is the European data processing network and management system for reporting and evaluation of suspected adverse reactions to medicines which have been authorised or being studied in clinical trials in the European Economic Area (EEA). The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network. The European EudraVigilance system deals with the: * Electronic exchange of Individual Case Safety Reports (ICSR, based on the ICH E2B specifications): ** EudraVigilance Clinical Trial Module (EVCTM) for reporting Suspected Unexpected Serious Adverse Reactions ( SUSARs). ** EudraVigilance Post-Authorisation Module (EVPM) for post-authorisation ICSRs. * Early detection of possible safety signals from marketed drugs for human use.CIOMS Working Group VIII. Practical aspects of signal detection in pharmacovigilance. Geneva: Council for International Organiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Society Of Pharmacovigilance, India
The Society of Pharmacovigilance, India (SoPI), is an Indian national non-profit scientific organisation, which aims at organizing training programmes and providing expertise in pharmacovigilance and enhance all aspects of the safe and proper use of medicines The International Society of Pharmacovigilance (ISoP) granted status of 'associated society' to Society of Pharmacovigilance India (SoPI). It is the second professional society in the world after ISoP. The founder of SoPI is KC Singhal. Annual Events * 14th Annual Conference of SoPI (SoPICON 2014), Aligarh, Indi* 19th Annual Conference of SoPI (SoPICON 2022), Online/Hybri Official Publication * Journal of Pharmacovigilance and Drug Safety (ISSN: 0972-8899 Office Bearers * Prof. KC Singhal (Patron) * Dr. Sandeep Agarwal (President) * Prof. Syed Ziaur Rahman (General Secretary) See also *Uppsala Monitoring Centre (WHO) *Council for International Organizations of Medical Sciences *EudraVigilance EudraVigilance (European Unio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)