|
Vedolizumab
Vedolizumab, sold under the brand name Entyvio, is a monoclonal antibody medication developed by Millennium Pharmaceuticals, Inc. for the treatment of ulcerative colitis and Crohn's disease. It binds to integrin α4β7 ( LPAM-1, lymphocyte Peyer's patch adhesion molecule 1, a dimer of Integrin alpha-4 and Integrin beta-7). Blocking the α4β7 integrin results in gut-selective anti-inflammatory activity. Medical use Ulcerative colitis and Crohn's disease Vedolizumab has been approved for use in adults with moderate to severe ulcerative colitis or Crohn's disease having a poor response to tumor necrosis factor (TNF) blockers or corticosteroids, or for those who are steroid-dependent. Checkpoint inhibitor colitis Vedolizumab may be used to treat steroid refractory checkpoint inhibitor induced colitis, if infliximab is ineffective or contraindicated. Clinical trials Ulcerative colitis Vedolizumab has been investigated in several studies in adult patients. Patients wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Checkpoint Inhibitor Induced Colitis
Checkpoint inhibitor induced colitis is an inflammatory condition affecting the colon (colitis), which is caused by cancer immunotherapy ( checkpoint inhibitor therapy). Symptoms typically consist of diarrhea, abdominal pain and rectal bleeding. Less commonly, nausea and vomiting may occur, which may suggest the present of gastroenteritis. The severity of diarrhea and colitis are graded based on the frequency of bowel movements and symptoms of colitis, respectively. The gold standard for the diagnosis of checkpoint inhibitor induced colitis is colonoscopy with evaluation of the terminal ileum. However, in most cases, a flexible sigmoidoscopy is sufficient. Infection should be ruled out with stool studies, including Clostridioides difficile, bacterial culture, ova and parasites. Symptoms of upper abdominal pain, nausea or vomiting warrant evaluation with upper endoscopy. Treatment of immune checkpoint inhibitor colitis is based on severity, as defined by the grade of diarrhea and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
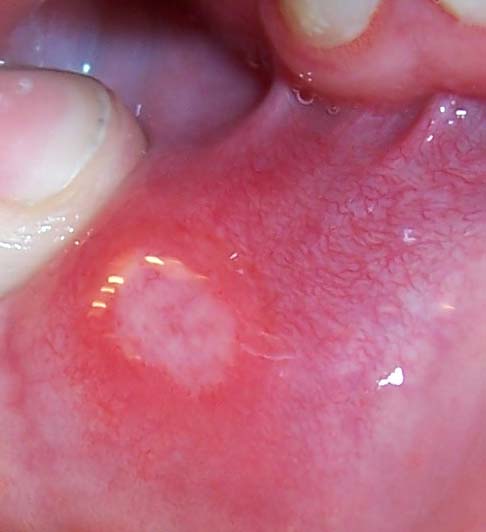
Ulcerative Colitis
Ulcerative colitis (UC) is a long-term condition that results in inflammation and ulcers of the colon and rectum. The primary symptoms of active disease are abdominal pain and diarrhea mixed with blood (hematochezia). Weight loss, fever, and anemia may also occur. Often, symptoms come on slowly and can range from mild to severe. Symptoms typically occur intermittently with periods of no symptoms between flares. Complications may include abnormal dilation of the colon ( megacolon), inflammation of the eye, joints, or liver, and colon cancer. The cause of UC is unknown. Theories involve immune system dysfunction, genetics, changes in the normal gut bacteria, and environmental factors. Rates tend to be higher in the developed world with some proposing this to be the result of less exposure to intestinal infections, or to a Western diet and lifestyle. The removal of the appendix at an early age may be protective. Diagnosis is typically by colonoscopy with tissue biopsies. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crohn's Disease
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea (which may be bloody if inflammation is severe), fever, abdominal distension, and weight loss. Complications outside of the gastrointestinal tract may include anemia, skin rashes, arthritis, inflammation of the eye, and fatigue. The skin rashes may be due to infections as well as pyoderma gangrenosum or erythema nodosum. Bowel obstruction may occur as a complication of chronic inflammation, and those with the disease are at greater risk of colon cancer and small bowel cancer. While the precise causes of Crohn's disease (CD) are unknown, it is believed to be caused by a combination of environmental, immune, and bacterial factors in genetically susceptible individuals. It results in a chronic inflammatory disorder, in which the body's immune system defends the gastrointestinal tract, possibly targeting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes. It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and thera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-retroviral Therapy
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same sex and opposite sex partners so long as the HIV-positive partner maintains an undetectable viral load. Treatment has been so successful that in many parts of the world, HIV has become a chronic condition in which progression to AIDS is increasingly rare. Anthony Fauci, head of the United States National Institute of Allergy and Infectious Diseases, has written, "With collective and resolute action now and a steadfas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhesus Macaques
The rhesus macaque (''Macaca mulatta''), colloquially rhesus monkey, is a species of Old World monkey. There are between six and nine recognised subspecies that are split between two groups, the Chinese-derived and the Indian-derived. Generally brown or grey in colour, it is in length with a tail and weighs . It is native to South, Central, and Southeast Asia and has the widest geographic range of all non-human primates, occupying a great diversity of altitudes and a great variety of habitats, from grasslands to arid and forested areas, but also close to human settlements. Feral colonies are found in the United States, thought to be either released by humans or escapees after hurricanes destroyed zoo and wildlife park facilities. The rhesus macaque is diurnal, arboreal, and terrestrial. It is mostly herbivorous, mainly eating fruit, but will also consume seeds, roots, buds, bark, and cereals. Studies show almost 100 different plant species in its diet. Rhesus macaques are gene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simian Immunodeficiency Virus
''Simian immunodeficiency virus'' (''SIV'') is a species of retrovirus that cause persistent infections in at least 45 species of non-human primates. Based on analysis of strains found in four species of monkeys from Bioko Island, which was isolated from the mainland by rising sea levels about 11,000 years ago, it has been concluded that SIV has been present in monkeys and apes for at least 32,000 years, and probably much longer. Virus strains from three of these primate species, SIVsmm in sooty mangabeys, SIVgor in gorillas and SIVcpz in chimpanzees, are believed to have crossed the species barrier into humans, resulting in HIV-2 and HIV-1 respectively, the two HIV viruses. The most likely route of transmission of HIV-1 to humans involves contact with the blood of chimps and gorillas that are often hunted for bushmeat in Africa. Four subtypes of HIV-1 (M, N, O, and P) likely arose through four separate transmissions of SIV to humans, and the resulting HIV-1 group M stra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NIAID
The National Institute of Allergy and Infectious Diseases (NIAID, ) is one of the 27 institutes and centers that make up the National Institutes of Health (NIH), an agency of the United States Department of Health and Human Services (HHS). NIAID's mission is to conduct basic and applied research to better understand, treat, and prevent infectious, immunologic, and allergic diseases. NIAID has on-campus laboratories in Maryland and Hamilton, Montana, and funds research conducted by scientists at institutions in the United States and throughout the world. NIAID also works closely with partners in academia, industry, government, and non-governmental organizations in multifaceted and multidisciplinary efforts to address emerging health challenges such as the H1N1/09 pandemic and the COVID-19 pandemic. History NIAID traces its origins to a small laboratory established in 1887 at the Marine Hospital on Staten Island, New York (now the Bayley Seton Hospital). Officials of the Mari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biologic License Application
A biologics license application (BLA) is defined by the U.S. Food and Drug Administration (FDA) as follows: The biologics license application is a request for permission to introduce, or deliver for introduction, a biologic product into interstate commerce (21 CFR 601.2). The BLA is regulated under 21 CFR 600 – 680. A BLA is submitted by any legal person or entity who is engaged in manufacture or an applicant for a license who takes responsibility for compliance with product and establishment standards. Form 356h specifies the requirements for a BLA. This includes: * Applicant information * Product/manufacturing information * Pre-clinical studies * Clinical studies * Labeling Some biological products are regulated by the Center for Drug Evaluation and Research (CDER) while others are regulated by Center for Biologics Evaluation and Research The Center for Biologics Evaluation and Research (CBER) is one of six main centers for the U.S. Food and Drug Administration (FDA), w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marketing Authorization Application
Marketing Authorisation Application (MAA) is an application submitted by a drug manufacturer seeking marketing authorisation, that is permission to bring a medicinal product (for example, a new medicine or generic medicine) to the market. MAA is part of the official procedure before the Medicines and Healthcare products Regulatory Agency in the United Kingdom and the Committee for Medicinal Products for Human Use of the European Medicines Agency, a specialised agency of the European Commission. In the United States The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ..., the equivalent process is called New Drug Application. References {{reflist Drug safety ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
