|
Vanadium(II) Chloride
Vanadium(II) chloride is the inorganic compound with the formula VCl2, and is the most reduced vanadium chloride. Vanadium(II) chloride is an apple-green solid that dissolves in water to give purple solutions. Preparation, properties, and related compounds Solid VCl2 is prepared by thermal decomposition of VCl3, which leaves a residue of VCl2:Young, R. C.; Smith, M. E. "Vanadium(II) Chloride" Inorganic Syntheses, 1953, volume IV, page 126-127. :2 VCl3 → VCl2 + VCl4 VCl2 dissolves in water to give the purple hexaaquo ion (H2O)6sup>2+. Evaporation of such solutions produces crystals of (H2O)6l2. Vanadium dichloride is used as a specialty reductant in organic chemistry. As an aqueous solution, it converts cyclohexylnitrate to cyclohexanone. It reduces phenyl azide into aniline Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Aquo Complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand ("homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands. Stoichiometry and structure Hexa-aquo complexes Most aquo complexes are mono-nuclear, with the general formula , with or 3; they have an octahedral structure. The water molecules function as Lewis bases, donating a pair of electrons to the metal ion and forming a dative covalent bond with it. Typical examples are listed in the following table. : Tutton's salts are crystalline compounds with the generic formula (where , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(II) Compounds
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( passivation) somewhat stabilizes the free metal against further oxidation. Spanish scientist Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by analyzing a new lead-bearing mineral he called "brown lead". Though he initially presumed its qualities were due to the presence of a new element, he was later erroneously convinced by French chemist Hippolyte Victor Collet-Descotils that the element was just chromium. Then in 1830, Nils Gabriel Sefström generated chlorides of vanadium, thus proving there was a new element, and named it "vanadium" after the Scandinavian goddess of beauty and fertility, Vanadís (Freyja). The name was based on the wide range of colors found in vanadium compounds. Del Rio's lead mineral w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline Aniline is an organic compound with the formula C6 H5 NH2. Consi .... It is an industrially significant Commodity chemicals, commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It Combustion, ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Azide
Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear organic azide, azide substituent bound to a phenyl group. The C−N=N angle is approximately 120°. Preparation Phenyl azide is prepared by the Diazonium compound, diazotization of phenylhydrazine with nitrous acid: :C6H5NHNH2 + HNO2 → C6H5N3 + 2 H2O Aryl iodides bearing electron-withdrawing substituents undergo metathesis with sodium azide in the presence of Cu(I), sodium ascorbate, and 1,2-Dimethylethylenediamine, N,N'-dimethylethane-1,2-diamine (DMEDA): :RC6H4I + NaN3 → RC6H4N3 + NaI It can also be prepared by condensation of benzenediazonium salt with toluenesulfonamide, followed by hydrolysis. Chemical reactions Phenyl azide cycloadds to alkenes and especially Azide alkyne Huisgen cycloaddition, alkynes, particularly those bearing electronegative substituents. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon. Production Cyclohexanone is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts: :C6H12 + O2 → (CH2)5CO + H2O This process forms cyclohexanol as a by-product, and this mixture, called "KA Oil" for ketone-alcohol oil, is the main feedstock for the production of adipic acid. The oxidation involves radicals and the hydroperoxide C6H11O2H as an intermediate. In some cases, purified cyclohexanol, obtained by hydration of cyclohexene, is the precursor. Alternatively, cyclohexanone can be produced by the partial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(II) Fluoride
Vanadium(II) fluoride is a fluoride of vanadium, with the chemical formula of VF2. It forms blue crystals. Preparation Vanadium(II) fluoride can be produced by the reduction of vanadium trifluoride by hydrogen in a hydrogen fluoride atmosphere at 1150 °C:Lothar Kolditz: ''Anorganische Chemie Teil 2''. VEB Deutscher Verlag der Wissenschaften, Berlin, 1980, S. 641. : Properties Physical properties Vanadium(II) fluoride crystallizes in the tetragonal crystal system with space group ''P''42/''mnm'' (No. 136). Its lattice constants are a = 480.4 pm and c = 323.7 pm.J. W. Stout, W. O. J. Boo: ''Crystalline vanadium (II) fluoride, VF2. Preparation, structure, heat capacity from 5 to 300 K and magnetic ordering''. In: ''The Journal of Chemical Physics''. 71, 1, 1979, S. 1–8, . Reactions Vanadium(II) fluoride is a strong reducing agent that can reduce nitrogen to hydrazine in the presence of magnesium hydroxide Magnesium hydroxide is the inorganic compound with the che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
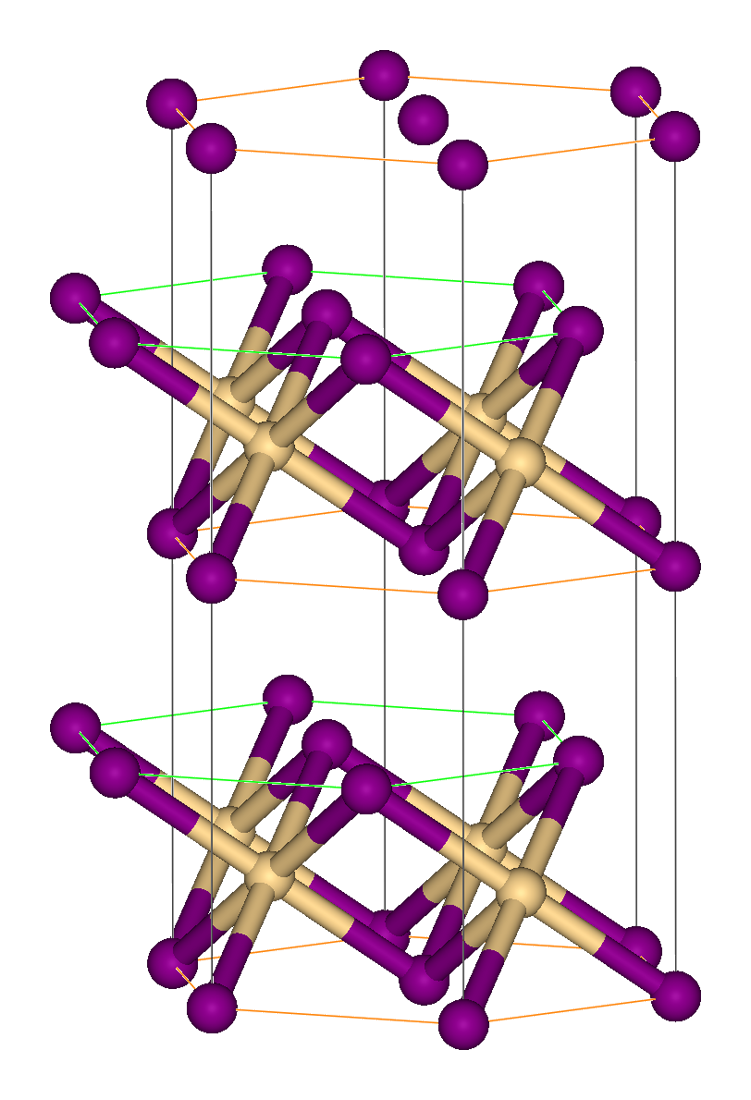
Vanadium(III) Chloride
Vanadium trichloride is the inorganic compound with the formula VCl3. This purple salt is a common precursor to other vanadium(III) complexes. Structure VCl3 has the common BiI3 structure, a motif that features hexagonally closest-packed chloride framework with vanadium ions occupying the octahedral holes. VBr3 and VI3 adopt the same structure, but VF3 features a structure more closely related to ReO3. VCl3 is paramagnetic and has two unpaired electrons. Preparation and reactions VCl3 is prepared by heating VCl4 at 160–170 °C under a flowing stream of inert gas, which sweeps out the Cl2. The bright red liquid converts to a purple solid. Heating of VCl3 decomposes with volatilization of VCl4, leaving VCl2. Upon heating under H2 at 675 °C (but less than 700 °C), VCl3 reduces to greenish VCl2. :: 2 VCl3 + H2 → 2 VCl2 + 2 HCl Comproportionation of vanadium trichloride and vanadium(V) oxides gives vanadium oxydichloride: :V2O5 + VOCl3 + 3 VCl3 → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_ion_in_aqueous_solution.jpg)



