|
Uranium Ditelluride
Uranium ditelluride is an inorganic compound with the formula UTe2. It was discovered to be an unconventional superconductor in 2018. * Superconductivity Superconductivity in UTe2 appears to be a consequence of triplet electrons spin-pairing. The material acts as a topological superconductor, stably conducting electricity without resistance even in high magnetic fields. It has superconducting transition temperature at Tc= 2K. Charge density waves (CDW) and pair density waves (PDW) have been described in UTe2, with the latest case being the first time it has been described in a p-wave superconductor. See also * Distrontium ruthenate a ''p''-wave triplet state superconductor candidate. * Helium-3 a spin-triplet superfluid * Ferromagnetic superconductor Ferromagnetic superconductors are materials that display intrinsic coexistence of ferromagnetism and superconductivity. They include UGe2, URhGe, and UCoGe. Evidence of ferromagnetic superconductivity was also reported ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. In nature, uranium is found as uranium-238 (99. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to the magnetic field. A permanent magnet's magnetic field pulls on ferromagnetic materials such as iron, and attracts or repels other magnets. In addition, a nonuniform magnetic field exerts minuscule forces on "nonmagnetic" materials by three other magnetic effects: paramagnetism, diamagnetism, and antiferromagnetism, although these forces are usually so small they can only be detected by laboratory equipment. Magnetic fields surround magnetized materials, and are created by electric currents such as those used in electromagnets, and by electric fields varying in time. Since both strength and direction of a magnetic field may vary with location, it is described mathematically by a function assigning a vector to each point of space, cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium(IV) Compounds
Uranous is the chemical term for the reduced tetrapositive cation of uranium that exhibits the valence U4+. It is one of the two common ionic states of uranium found in nature, the other being the oxidised hexapositive ion called uranyl. Uranous compounds are usually unstable; they revert to the oxidised form on exposure to air. Examples of these compounds include uranium tetrachloride () and uranium tetrafluoride (), which are important in molten salt reactor applications, and uranium dioxide (), a common form of nuclear fuel. The solvated ion is normally not present in water. Most of the compounds like are better described with the covalent bond than an ionic bond. Minerals containing the uranous ion are more subdued in colour, typically brown or black, and occur in reducing environments. Common uranous minerals include uraninite and coffinite Coffinite is a uranium-bearing silicate mineral with formula: U(SiO4)1−x(OH)4x. It occurs as black incrustations, dark to pale-br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reentrant Superconductivity
In physics, reentrant superconductivity is an effect observed in systems that lie close to the boundary between ferromagnetic and superconducting. By its very nature (normal) superconductivity (condensation of electrons into the BCS ground state) cannot exist together with ferromagnetism (condensation of electrons into the same spin state, all pointing in the same direction). Reentrance is when while changing a continuous parameter, superconductivity is first observed, then destroyed by the ferromagnetic order, and later reappears. An example is the changing of the thickness of the ferromagnetic layer in a bilayer of a superconductor and a ferromagnet. At a certain thickness superconductivity is destroyed by the Andreev reflected electrons in the ferromagnet, but if the thickness increases, this effect disappears again. Another example are materials with a Curie temperature below the superconducting transition temperature. When cooling, first superconducting order appears in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetic Superconductor
Ferromagnetic superconductors are materials that display intrinsic coexistence of ferromagnetism and superconductivity. They include UGe2, URhGe, and UCoGe. Evidence of ferromagnetic superconductivity was also reported for ZrZn2 in 2001, but later reports question these findings. These materials exhibit superconductivity in proximity to a magnetic quantum critical point. The nature of the superconducting state in ferromagnetic superconductors is currently under debate. Early investigations studied the coexistence of conventional ''s''-wave superconductivity with itinerant ferromagnetism. However, the scenario of spin-triplet pairing soon gained the upper hand. A mean-field model for coexistence of spin-triplet pairing and ferromagnetism was developed in 2005. These models consider uniform coexistence of ferromagnetism and superconductivity, i.e. the same electrons which are both ferromagnetic and superconducting at the same time. Another scenario where there is an interplay betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron (the most common isotope, helium-4, having two protons and two neutrons in contrast). Other than protium (ordinary hydrogen), helium-3 is the only stable isotope of any element with more protons than neutrons. Helium-3 was discovered in 1939. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. Helium-3 is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some of the helium-3 found in the terrestrial atmosphere is also an artifact of atmospheric and underwater nuclear weapons testing. Much speculation has been made over the possibility of helium-3 as a future energy source. Unlike most nuclear fusion reactions, the fusion of helium-3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distrontium Ruthenate
Distrontium ruthenate, also known as strontium ruthenate, is an oxide of strontium and ruthenium with the chemical formula Sr2RuO4. It was the first reported perovskite superconductor that did not contain copper. Strontium ruthenate is structurally very similar to the high-temperature cuprate superconductors, and in particular, is almost identical to the lanthanum doped superconductor (La, Sr)2CuO4. However, the transition temperature for the superconducting phase transition is 0.93 K (about 1.5 K for the best sample), which is much lower than the corresponding value for cuprates. Superconductivity Superconductivity in SRO was first observed by Yoshiteru Maeno et al. Unlike the cuprate superconductors, SRO displays superconductivity in the absence of doping. The superconducting order parameter in SRO exhibits signatures of time-reversal symmetry breaking, and hence, it can be classified as an unconventional superconductor. Sr2RuO4 is believed to be a fairly two-dimensio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Density Wave
A charge density wave (CDW) is an ordered quantum fluid of electrons in a linear chain compound or layered crystal. The electrons within a CDW form a standing wave pattern and sometimes collectively carry an electric current. The electrons in such a CDW, like those in a superconductor, can flow through a linear chain compound en masse, in a highly correlated fashion. Unlike a superconductor, however, the electric CDW current often flows in a jerky fashion, much like water dripping from a faucet due to its electrostatic properties. In a CDW, the combined effects of pinning (due to impurities) and electrostatic interactions (due to the net electric charges of any CDW kinks) likely play critical roles in the CDW current's jerky behavior, as discussed in sections 4 & 5 below. Most CDW's in metallic crystals form due to the wave-like nature of electrons – a manifestation of quantum mechanical wave-particle duality – causing the electronic charge density to become spatially modula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.Anderson, Don L.; "Chemical Composition of the Mantle" in ''Theory of the Earth'', pp. 147-175 Tellurium-bearing compounds were first discovered in 1782 in a gold mine in Kleinschlatten, Transylvania (now Zlatna, Romania) by Austrian mineralogist Franz-Joseph Müller von Reichenstein, although it was Martin Heinrich Klaproth who named the new element in 1798 after the Latin 'earth'. Gold telluride minerals ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
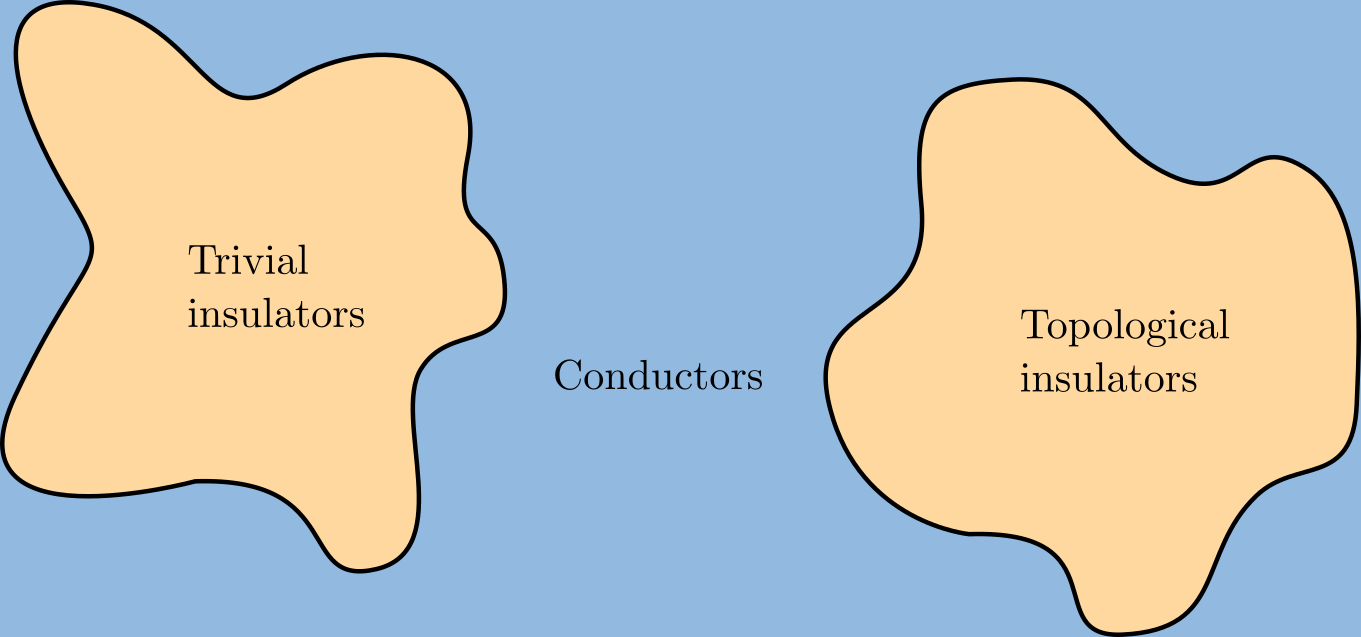
Topological Insulator
A topological insulator is a material whose interior behaves as an electrical insulator while its surface behaves as an electrical conductor, meaning that electrons can only move along the surface of the material. A topological insulator is an insulator for the same reason a "trivial" (ordinary) insulator is: there exists an energy gap between the valence and conduction bands of the material. But in a topological insulator, these bands are, in an informal sense, "twisted", relative to a trivial insulator. The topological insulator cannot be continuously transformed into a trivial one without untwisting the bands, which closes the band gap and creates a conducting state. Thus, due to the continuity of the underlying field, the border of a topological insulator with a trivial insulator (including vacuum, which is topologically trivial) is forced to support a conducting state. Since this results from a global property of the topological insulator's band structure, local (symmetry- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exchange Interaction
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classical force, it is not a true force as it lacks a force carrier. The effect is due to the wave function of indistinguishable particles being subject to exchange symmetry, that is, either remaining unchanged (symmetric) or changing sign (antisymmetric) when two particles are exchanged. Both bosons and fermions can experience the exchange interaction. For fermions, this interaction is sometimes called Pauli repulsion and is related to the Pauli exclusion principle. For bosons, the exchange interaction takes the form of an effective attraction that causes identical particles to be found closer together, as in Bose–Einstein condensation. The exchange interaction alters the expectation value of the distance when the wave functions of two or more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_0.002_K_region.png)