|
Unimolecular Ion Decomposition
Unimolecular ion decomposition is the fragmentation of a gas phase ion in a reaction with a molecularity of one. Ions with sufficient internal energy may fragment in a mass spectrometer, which in some cases may degrade the mass spectrometer performance, but in other cases, such as tandem mass spectrometry, the fragmentation can reveal information about the structure of the ion. Wahrhaftig diagram A Wahrhaftig diagram (named after Austin L. Wahrhaftig) illustrates the relative contributions in unimolecular ion decomposition of direct fragmentation and fragmentation following rearrangement. The x-axis of the diagram represents the internal energy of the ion. The lower part of the diagram shows the logarithm of the rate constant ''k'' for unimolecular dissociation whereas the upper portion of the diagram indicates the probability of forming a particular product ion. The green trace in the lower part of the diagram indicates the rate of the rearrangement reaction given by :ABCD+ -> + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecularity
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coefficients of reactants in the elementary reaction with effective collision ( sufficient energy) and correct orientation. Depending on how many molecules come together, a reaction can be unimolecular, bimolecular or even trimolecular. The kinetic order of any elementary reaction or reaction step is ''equal'' to its molecularity, and the rate equation of an elementary reaction can therefore be determined by inspection, from the molecularity. The kinetic order of a complex (multistep) reaction, however, is not necessarily equal to the number of molecules involved. The concept of molecularity is only useful to describe elementary reactions or steps. Unimolecular reactions In a unimolecular reaction, a single molecule rearranges atoms, forming ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activated Complex
In chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry (IUPAC) as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col (saddle point) of a potential energy surface". In other words, it refers to a collection of intermediate structures in a chemical reaction that persist while bonds are breaking and new bonds are forming. It therefore represents not one defined state, but rather a range of transient configurations that a collection of atoms passes through in between clearly defined products and reactants. It is the subject of transition state theory - also known as activated complex theory - which studies the kinetics of reactions that pass through a defined intermediate state with standard Gibbs energy of activation . The state represented by the double dagger symbol is known as the transition state and represents the exact configuration that has an equal probability of forming eith ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tandem Mass Spectrometry
Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more mass analyzers are coupled together using an additional reaction step to increase their abilities to analyse chemical samples. A common use of tandem MS is the analysis of biomolecules, such as proteins and peptides. The molecules of a given sample are ionized and the first spectrometer (designated MS1) separates these ions by their mass-to-charge ratio (often given as m/z or m/Q). Ions of a particular m/z-ratio coming from MS1 are selected and then made to split into smaller fragment ions, e.g. by collision-induced dissociation, ion-molecule reaction, or photodissociation. These fragments are then introduced into the second mass spectrometer (MS2), which in turn separates the fragments by their m/z-ratio and detects them. The fragmentation step makes it possible to identify and separate ions that have very similar m/z-ratios in regular mass spectrometers. Struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RRKM Theory
The Rice–Ramsperger–Kassel–Marcus (RRKM) theory is a theory of chemical reactivity. It was developed by Rice and Ramsperger in 1927 and Kassel in 1928 (RRK theory) and generalized (into the RRKM theory) in 1952 by Marcus who took the transition state theory developed by Eyring in 1935 into account. These methods enable the computation of simple estimates of the unimolecular reaction rates The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ... from a few characteristics of the potential energy surface. Assumption Assume that the molecule consists of harmonic oscillators, which are connected and can exchange energy with each other. * Assume the possible excitation energy of the molecule to be , which enables the reaction to occur. * The rate of intra-molecular energy dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State Theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ''H''‡, also written Δ‡''H''ɵ), the standard entropy of activation (Δ''S''‡ or Δ‡''S''ɵ), and the standard Gibbs energy of activation (Δ''G''‡ or Δ‡''G''ɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest ''at the transition state''; Δ''H''‡ is the difference between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastability
In chemistry and physics, metastability denotes an intermediate Energy level, energetic state within a dynamical system other than the system's ground state, state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effect
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it. In the International System of Units (SI), the unit of measurement for enthalpy is the joule. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
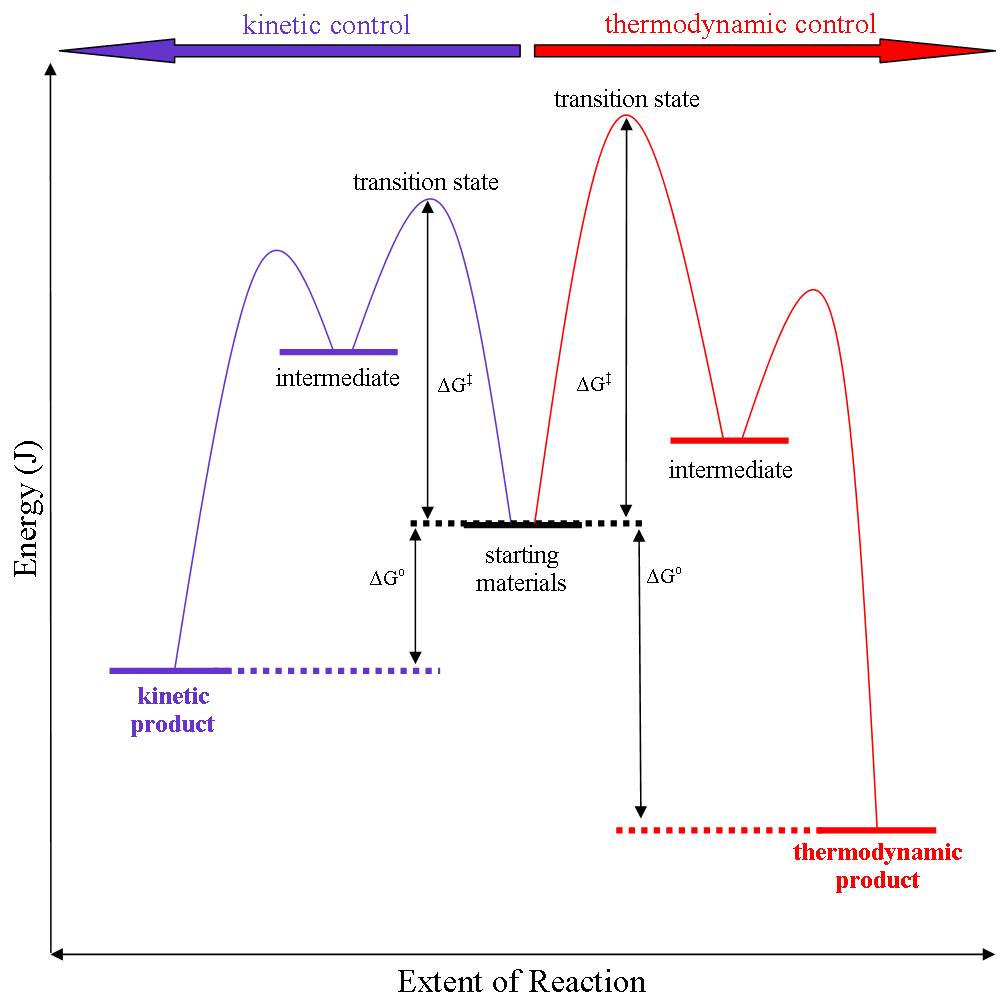
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the conversion (chemistry), selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ABCD Transition States
ABCD is a list of the first four letters in the English alphabet. It may also refer to: Film * ''ABCD'' (film), a 2005 Tamil romance film * ''ABCD 2'', a 2015 Indian dance film * '' ABCD: American-Born Confused Desi (2013 film)'', a 2013 Malayalam comedy film * '' ABCD: American Born Confused Desi (2019 film)'', a 2019 Telugu drama film * '' ABCD: Any Body Can Dance'', a 2013 Bollywood film Medicine * ABC (medicine), also known as ABCD, a mnemonic for steps in resuscitation * ABCD rating, a staging system for prostate cancer * ABCD syndrome, a genetic disorder * ABCD1, a protein * ABCD2 score, a score for determining the risk of stroke after a transient ischemic attack Science * ABCD Schema, a highly structured data exchange and access model for taxon occurrence data (specimens, observations, etc. of living organisms) * ABCD matrix analysis, a type of ray tracing technique used in the design of some optical systems * ''ABCD''-parameters, a type of properties describing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zero Point Energy
Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty principle. Therefore, even at absolute zero, atoms and molecules retain some vibrational motion. Apart from atoms and molecules, the empty space of the vacuum also has these properties. According to quantum field theory, the universe can be thought of not as isolated particles but continuous fluctuating fields: matter fields, whose quanta are fermions (i.e., leptons and quarks), and force fields, whose quanta are bosons (e.g., photons and gluons). All these fields have zero-point energy. These fluctuating zero-point fields lead to a kind of reintroduction of an aether in physics since some systems can detect the existence of this energy. However, this aether cannot be thought of as a physical medium if it is to be Lorentz invaria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |