|
Uncaria Tomentosa
''Uncaria tomentosa'' is a woody vine found in the tropical jungles of South and Central America. It is known as cat's claw or uña de gato in Spanish because of its claw-shaped thorns. The plant root bark is used in herbalism for a variety of ailments, and is sold as a dietary supplement. There is no clinical evidence that cat's claw is effective for treating any human disease. Description ''Uncaria tomentosa'' is a liana deriving its name from hook-like thorns that resemble the claws of a cat. ''U. tomentosa'' can grow to a length of up to 30 m (100 ft), climbing by means of these thorns. The leaves are elliptic with a smooth edge, and grow in opposing pairs. Cat's claw is indigenous to the Amazon rainforest, with its habitat being restricted primarily to the tropical areas of South and Central America. Taxonomy There are two species of cat's claw commonly used in North America and Europe, ''Uncaria tomentosa'' and ''Uncaria guianensis'', having different prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytochemical
Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes . Some phytochemicals have been used as poisons and others as traditional medicine. As a term, ''phytochemicals'' is generally used to describe plant compounds that are under research with unestablished effects on health, and are not scientifically defined as essential nutrients. Regulatory agencies governing food labeling in Europe and the United States have provided guidance for industry to limit or prevent health claims about phytochemicals on food product or nutrition labels. Definition Phytochemicals are chemicals of plant origin. Phytochemicals (from Greek ''phyto'', meaning "plant") are chemicals produced by plants through primary or secondary metabolism. They generally have biological activity in the plant host and play a role in plant growth or defense against competitors, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
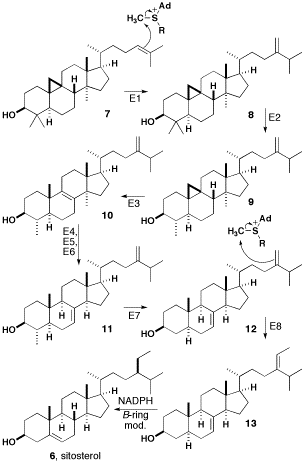
Beta-sitosterol
β-sitosterol (beta-sitosterol) is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols. Natural occurrences and food β-sitosterol is widely distributed in the plant kingdom. It is found in vegetable oil, nuts, avocados, and derived prepared foods such as salad dressings. Human research β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH) and blood cholesterol levels. Genetic disorder While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols. Precursor of anabolic steroid boldenone Being a steroid, β-sitosterol is a precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to indu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhynchophylline
Rhynchophylline is an alkaloid found in certain ''Uncaria'' species (Rubiaceae), notably '' Uncaria rhynchophylla'' and '' Uncaria tomentosa''. It also occurs in the leaves of ''Mitragyna speciosa'' (kratom), a tree native to Thailand. Chemically, it is related to the alkaloid mitragynine. Rhynchophylline is a non-competitive NMDA antagonist (IC50 = 43.2 μM) and a calcium channel blocker. ''Uncaria'' species have had a variety of uses in traditional herbal medicine, such as for lightheadedness, convulsions, numbness, and hypertension. These uses have been associated with the presence of rhynchophylline and have encouraged its investigation as a drug candidate for several cardiovascular and central nervous system diseases; however, few clinically relevant studies have been conducted. See also *Ibotenic acid * Mitragynine pseudoindoxyl *''Proboscidea parviflora ''Proboscidea parviflora'' is a species of flowering plant in the family Martyniaceae known by the common names ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechin
Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids. The name of the catechin chemical family derives from ''catechu'', which is the tannic juice or boiled extract of ''Mimosa catechu'' (''Acacia catechu'' L.f). Chemistry Catechin possesses two benzene ring Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atom ...s (called the A- and B-rings) and a dihydropyran heterocycle (the C-ring) with a hydroxyl group on carbon 3. The A-ring is similar to a resorcinol moiety while the B-ring is similar to a catechol moiety. There are two chirality (chemistry), chiral centers on the molecule on carbons 2 and 3. Therefore, it has four diastereoisomers. Two of the isomers are in trans configura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyphenol
Polyphenols () are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments. Etymology The name derives from the Ancient Greek word (''polus'', meaning "many, much") and the word phenol which refers to a chemical structure formed by attaching to an aromatic benzenoid (phenyl) ring to a hydroxyl (-OH) group as is found in alcohols (hence the ''-ol'' suffix). The term polyphenol has been in use at least since 1894. Definition The term polyphenol is not well-defined, but is generally agreed that they are natural products "having a polyphenol structure (i.e., several hydroxyl groups on aromatic rings)" including four principal classes: "phenolic acids, flavonoids, stilbenes, and lignans". *Flavonoids include flavones, flavonols, flavanols, f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tannin
Tannins (or tannoids) are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids. The term ''tannin'' (from Anglo-Norman ''tanner'', from Medieval Latin ''tannāre'', from ''tannum'', oak bark) refers to the use of oak and other bark in tanning animal hides into leather. By extension, the term ''tannin'' is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with various macromolecules. The tannin compounds are widely distributed in many species of plants, where they play a role in protection from predation (acting as pesticides) and might help in regulating plant growth. The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit, red wine or tea. Likewise, the destruction or modification of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' oft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (fats in the broader sense of the term). Types Sterols of plants are called ''phytosterols'' and sterols of animals are called ''zoosterols''. The most importa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proanthocyanidin
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins. Proanthocyanidins were discovered in 1947 by Jacques Masquelier, who developed and patented techniques for the extraction of oligomeric proanthocyanidins from pine bark and grape seeds. Often associated with prevention of urinary tract infections (UTIs) by consuming cranberries, grape seeds or red wine, proanthocyanidins have not been conclusively shown as effective for preventing or treating UTIs. Distribution in plants Proanthocyanidins, including the lesser bioactive and bioavailable polymers (four or more catechins), represent a group of condensed flavan-3-ols, such as procyanidins, prodelphinidins and propelargonidins. They can be found in many plant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can lsoconfer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids. A few common examples include: * lactic acid * acetic acid * formic acid * citric acid * oxalic acid * uric acid * malic acid * tartaric acid Characteristics In general, organic acids are weak acids and do not dissociate completely in water, whereas the strong mineral acids do. Lower molecular mass organic acids such as formic and lactic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |