|
Thionin
Thionins are a family of small proteins found solely in higher plants. Typically, a thionin consists of 45–48 amino acid residues. 6–8 of these are cysteine forming 3–4 disulfide bonds. They include phoratoxins and viscotoxins. Alpha- and beta- thionins are related to each other. The gamma thionins have a superficially similar structure but are an unrelated class of protein, now called plant defensins. Activity The proteins are toxic to animal cells, presumably attacking the cell membrane and rendering it permeable: this results in the inhibition of sugar uptake and allows potassium and phosphate ions, proteins, and nucleotides to leak from cells. Thionins are mainly found in seeds where they may act as a defence against consumption by animals. A barley (''Hordeum vulgare'') leaf thionin that is highly toxic to plant pathogens and is involved in the mechanism of plant defence against microbial infections has also been identified. The hydrophobic protein crambin from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Thionin
Plant defensins (formerly ''gamma-thionins)'' are a family of small, cysteine-rich defensins found in plants that serve to defend them against pathogens and parasites. History The first plant defensins were discovered in barley and wheat in 1990 and were initially designated as a γ-thionins. In 1995 the name was changed to 'plant defensin' when it was identified that they are evolutionarily unrelated to other thionins and were more similar to defensins from insects and mammals. Function Plant defensins are a large component of the plant innate immune system. A plant genome typically contains large numbers of different defensin genes that vary in their efficacies against different pathogens and the amount they are expressed in different tissues. Antimicrobial activity The modes of action of different defensins depends on the type of fungus they are interacting with. Most characterized plant defensins are antimicrobial peptides. Both antifungal and antibacterial plant def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Thionin
Plant defensins (formerly ''gamma-thionins)'' are a family of small, cysteine-rich defensins found in plants that serve to defend them against pathogens and parasites. History The first plant defensins were discovered in barley and wheat in 1990 and were initially designated as a γ-thionins. In 1995 the name was changed to 'plant defensin' when it was identified that they are evolutionarily unrelated to other thionins and were more similar to defensins from insects and mammals. Function Plant defensins are a large component of the plant innate immune system. A plant genome typically contains large numbers of different defensin genes that vary in their efficacies against different pathogens and the amount they are expressed in different tissues. Antimicrobial activity The modes of action of different defensins depends on the type of fungus they are interacting with. Most characterized plant defensins are antimicrobial peptides. Both antifungal and antibacterial plant def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phoratoxin
Phoratoxins are a group of peptide toxins that belong to the family of thionins, a subdivision of small plant toxins (5000 kD MW). Phoratoxins are proteins present in the leaves and branches of the ''Phoradendron'', commonly known as the American variant of the mistletoe, a plant commonly used as decoration during the festive season. The berries of the mistletoe do not contain phoratoxins, making them less toxic compared to other parts of the plant. The toxicity of the mistletoe is dependent on the host tree, since mistletoe is known to be a semi-parasite. The host tree provides fixed inorganic nitrogen compounds necessary for the mistletoe to synthesize phoratoxins. Viscotoxins are similar plant thionins produced from the leaves and stems of the European mistletoe (''Viscum album''). History The history of phoratoxin is filled with myths, legends, and other magical stories. Mistletoe is a semi-parasitic plant occasionally using oak trees as their host. To historic peoples such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscotoxin
Phoratoxins are a group of peptide toxins that belong to the family of thionins, a subdivision of small plant toxins (5000 kD MW). Phoratoxins are proteins present in the leaves and branches of the ''Phoradendron'', commonly known as the American variant of the mistletoe, a plant commonly used as decoration during the festive season. The berries of the mistletoe do not contain phoratoxins, making them less toxic compared to other parts of the plant. The toxicity of the mistletoe is dependent on the host tree, since mistletoe is known to be a semi-parasite. The host tree provides fixed inorganic nitrogen compounds necessary for the mistletoe to synthesize phoratoxins. Viscotoxins are similar plant thionins produced from the leaves and stems of the European mistletoe (''Viscum album''). History The history of phoratoxin is filled with myths, legends, and other magical stories. Mistletoe is a semi-parasitic plant occasionally using oak trees as their host. To historic peoples such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crambin
Crambin is a small seed storage protein from the Abyssinian cabbage. It belongs to thionins Thionins are a family of small proteins found solely in higher plants. Typically, a thionin consists of 45–48 amino acid residues. 6–8 of these are cysteine forming 3–4 disulfide bonds. They include phoratoxins and viscotoxins. Alpha- and .... It has 46 residues (amino acids). It has been extensively studied by X-ray crystallography since its crystals are unique and diffract to a resolution of 0.48 Å. Neutron scattering measurements are available also at a resolution of 1.1 Å.; References {{reflist Proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bonds
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of protein, proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peripheral Membrane Proteins
Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins. The reversible attachment of proteins to biological membranes has shown to regulate cell signaling and many other important cellular events, through a variety of mechanisms. For example, the close association between many enzy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crambin
Crambin is a small seed storage protein from the Abyssinian cabbage. It belongs to thionins Thionins are a family of small proteins found solely in higher plants. Typically, a thionin consists of 45–48 amino acid residues. 6–8 of these are cysteine forming 3–4 disulfide bonds. They include phoratoxins and viscotoxins. Alpha- and .... It has 46 residues (amino acids). It has been extensively studied by X-ray crystallography since its crystals are unique and diffract to a resolution of 0.48 Å. Neutron scattering measurements are available also at a resolution of 1.1 Å.; References {{reflist Proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Higher Plants
Vascular plants (), also called tracheophytes () or collectively Tracheophyta (), form a large group of land plants ( accepted known species) that have lignified tissues (the xylem) for conducting water and minerals throughout the plant. They also have a specialized non-lignified tissue (the phloem) to conduct products of photosynthesis. Vascular plants include the clubmosses, horsetails, ferns, gymnosperms (including conifers), and angiosperms (flowering plants). Scientific names for the group include Tracheophyta, Tracheobionta and Equisetopsida ''sensu lato''. Some early land plants (the rhyniophytes) had less developed vascular tissue; the term eutracheophyte has been used for all other vascular plants, including all living ones. Historically, vascular plants were known as "higher plants", as it was believed that they were further evolved than other plants due to being more complex organisms. However, this is an antiquated remnant of the obsolete scala naturae, and the term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
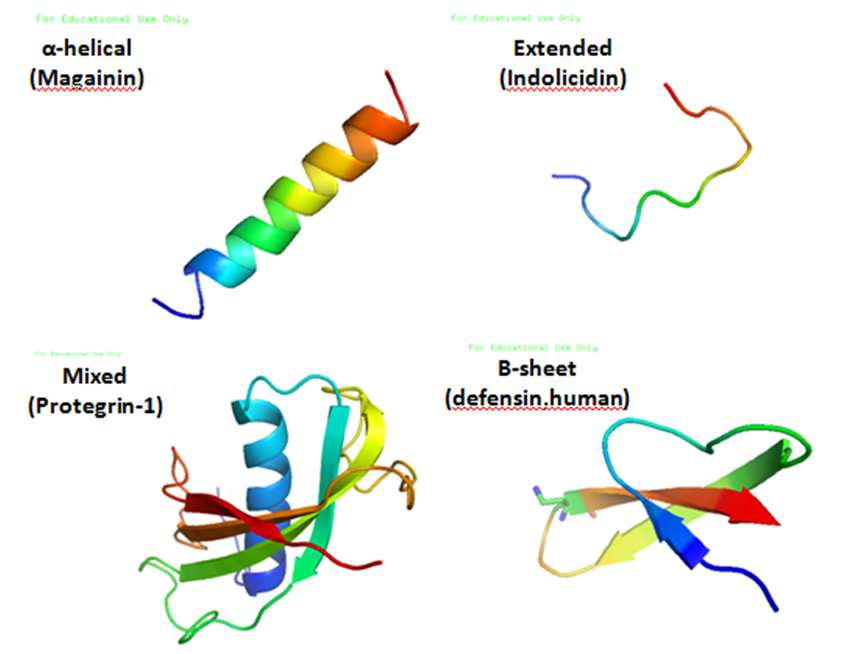
Antimicrobial Peptide
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |