|
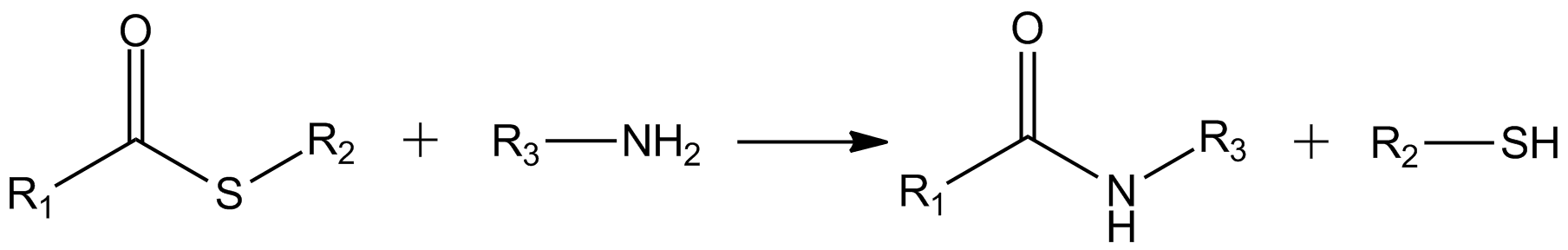
Thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the ''thio-'' prefix. They are the product of esterification between a carboxylic acid () and a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. Synthesis The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol: :RSNa + R'COCl -> R'COSR + NaCl Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: :CH3COSK + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenic acid, pantothenate (vitamin B5), and adenosine triphosphate (ATP). In acetyl-CoA, its acetyl form, coenzyme A is a highly versatile molecule, serving metabolic functions in both the Anabolism, anabolic and Catabolism, catabolic pathways. Acetyl-CoA is utilised in the post-translational regulation and allosteric regulation of pyruvate dehydrogenase and carboxylase to maintain and support the partition of Pyruvic acid, pyruvate synthesis and degradation. Discovery of structure Coenzyme A was identified by Fritz Lipmann in 1946, who also later gave it its name. Its structure was determined during the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Thioacetate
Potassium thioacetate is an organosulfur compound and a salt with the formula . This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives. Synthesis and reactions Potassium thioacetate, which is commercially available, can be prepared by combining acetyl chloride and potassium hydrogen sulfide: :CH3COCl + 2 KSH -> KCl + CH3COSK + H2S It arises also by the neutralization of thioacetic acid with potassium hydroxide. Use in preparation of thiols In a common application, potassium thioacetate is combined with alkylating agents to give thioacetate esters (X = halide): :CH3COSK + RX -> CH3COSR + KX Hydrolysis of these esters affords thiol In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...s: :CH3COSR + H2O -> CH3CO2H + RSH The thioace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocarboxylic Acid
In organic chemistry, thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a Thioketone, thione form () and a thiol form (). These are sometimes also referred to as "carbothioic ''O''-acid" and "carbothioic ''S''-acid" respectively. Of these the thiol form is most common (e.g. thioacetic acid). A naturally occurring thiocarboxylic acid is pyridine-2,6-dicarbothioic acid, a siderophore. Synthesis Thiocarboxylic acids are typically prepared by salt metathesis from the acid chloride, as in the following conversion of benzoyl chloride to thiobenzoic acid using potassium hydrosulfide according to the following idealized equation: :C6H5C(O)Cl + KSH -> C6H5C(O)SH + KCl Reactions At neutral pH, thiocarboxylic acids are fully ionized. Thiocarboxylic acids are about 100 times more acidic than the analogous carboxylic acids. For PhC(O)SH pKa = 2.48 vs 4.20 for benzoic acid, PhC( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioacetic Acid
Thioacetic acid is an organosulfur compound with the molecular formula . It is the sulfur analogue of acetic acid (), as implied by the ''thio-'' prefix. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the introduction of thiol groups () in molecules. Synthesis and properties Thioacetic acid is prepared by the reaction of acetic anhydride with hydrogen sulfide: :(CH3C(O))2O + H2S -> CH3C(O)SH + CH3C(O)OH It has also been produced by the action of phosphorus pentasulfide on glacial acetic acid, followed by distillation. :CH3C(O)OH + P2S5 -> CH3C(O)SH + P2OS4 Thioacetic acid is typically contaminated by acetic acid. The compound exists exclusively as the thiol tautomer, consistent with the strength of the double bond. Reflecting the influence of hydrogen-bonding, the boiling point (93 °C) and melting points are 20 and 75 K lower than those for acetic acid. Reactivity Acidity With a p''K''a near 3.4, thioacetic acid is about 15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanephosphonic Acid Anhydride
Propanephosphonic acid anhydride (PPAA, T3P) is an anhydride of propane phosphonic acid. Its structure is a cyclic trimer, with a phosphorus–oxygen core and propyl groups and additional oxygens attached. The chemical is a useful reagent for peptide synthesis reactions, where it activates the carboxylic acid partner for subsequent reaction with amines. It is commercially available as 50 % solution in DMF or ethyl acetate Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ... as a slightly yellow mixture. References Reagents for organic chemistry Phosphonates {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid. Preparation Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopentanone: :(CH2)4(CO2H)2 → (CH2)4CO + H2O + CO2 Uses Cyclopentanone is common precursor to fragrances, especially those related to jasmine and jasmone. Examples include 2-pentyl- and 2-heptylcyclopentanone.Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim. It is a versatile synthetic intermediate, being a precursor to cyclopentobarbital. Cyclopentanone is also used to make cyclopentamine, the pesticide pencycuron, and pentethylcyclanone Pentethylcyclanone is an antitussive medication A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid. In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R'. Organic acid anhydrides often form when one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction. In inorganic chemistry, an acid anhydride refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base to form a salt. Nomenclature The nomenclature of organic acid anhydrides is derived from the names of the constituent carboxylic acids which underwent dehydration to form the compound. In symmetrical acid anhydrides, where only one constituent carboxylic acid was used to form the compound (such as the dehydration of propanoic acid, 2CH3CH2COOH → CH3CH2C(O)OC(O)CH2CH3 + H2O), only the prefix of the original carboxylic acid is used and the suffix "anhydride" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (α-lactones and β-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered. Nomenclature Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |