|
Thermal Physics
Thermal physics is the combined study of thermodynamics, statistical mechanics, and kinetic theory of gases. This umbrella-subject is typically designed for physics students and functions to provide a general introduction to each of three core heat-related subjects. Other authors, however, define thermal physics loosely as a summation of only thermodynamics and statistical mechanics. Thermal physics can be seen as the study of systems with a large number of atoms. It unites thermodynamics and statistical mechanics. Overview Thermal physics, generally speaking, is the study of the statistical nature of physical systems from an energetic perspective. Starting with the basics of heat and temperature, thermal physics analyzes the first law of thermodynamics and second law of thermodynamics from the statistical perspective, in terms of the number of microstates corresponding to a given macrostate. In addition, the concept of entropy is studied via quantum theory. A central t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
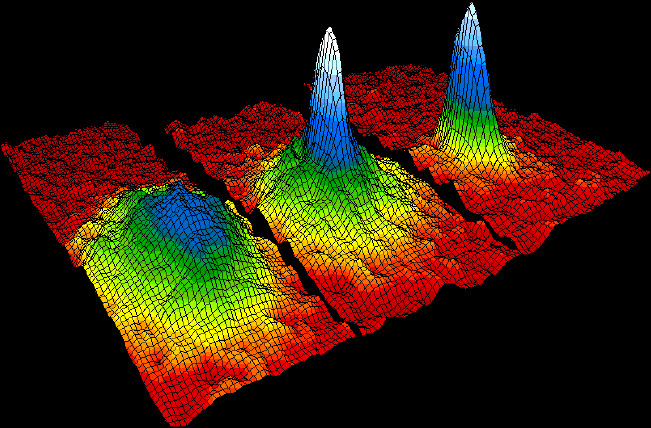
Bose Einstein Condensate
Bose may refer to: * Bose (crater), a lunar crater. * ''Bose'' (film), a 2004 Indian Tamil film starring Srikanth and Sneha * Bose (surname), a surname (and list of people with the name) * Bose (given name), a given name * Bose, Italy, a ''frazione'' in Magnano, Province of Biella ** Bose Monastic Community, a monastic community in the village * Bose, Poland * Bose Corporation, an audio company * Bose Ogulu, Nigerian manager of Burna Boy * Baise, or Bose, a prefecture-level city in Guangxi, China * '' Bose: The Forgotten Hero'', a 2004 Indian film about Subhas Chandra Bose * '' Bose: Dead/Alive'', a web series about Subhas Chandra Bose See also * * Boise (other) * Bos (other) * Bose–Einstein (other), articles relating to Bose–Einstein statistics and its consequences * Boson (other) A boson is a particle that has integer spin. Boson may also refer to: * John Boson, woodworker * John Boson (writer) (1655–1730), writer in the C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fermion
In particle physics, a fermion is a subatomic particle that follows Fermi–Dirac statistics. Fermions have a half-integer spin (spin 1/2, spin , Spin (physics)#Higher spins, spin , etc.) and obey the Pauli exclusion principle. These particles include all quarks and leptons and all composite particles made of an even and odd, odd number of these, such as all baryons and many atoms and atomic nucleus, nuclei. Fermions differ from bosons, which obey Bose–Einstein statistics. Some fermions are elementary particles (such as electrons), and some are composite particles (such as protons). For example, according to the spin-statistics theorem in Theory of relativity, relativistic quantum field theory, particles with integer Spin (physics), spin are bosons. In contrast, particles with half-integer spin are fermions. In addition to the spin characteristic, fermions have another specific property: they possess conserved baryon or lepton quantum numbers. Therefore, what is usually referr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Information Theory
Information theory is the mathematical study of the quantification (science), quantification, Data storage, storage, and telecommunications, communication of information. The field was established and formalized by Claude Shannon in the 1940s, though early contributions were made in the 1920s through the works of Harry Nyquist and Ralph Hartley. It is at the intersection of electronic engineering, mathematics, statistics, computer science, Neuroscience, neurobiology, physics, and electrical engineering. A key measure in information theory is information entropy, entropy. Entropy quantifies the amount of uncertainty involved in the value of a random variable or the outcome of a random process. For example, identifying the outcome of a Fair coin, fair coin flip (which has two equally likely outcomes) provides less information (lower entropy, less uncertainty) than identifying the outcome from a roll of a dice, die (which has six equally likely outcomes). Some other important measu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heat Transfer Physics
Heat transfer physics describes the kinetics of energy storage, transport, and energy transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is thermal energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is different made (converted) among various carriers. The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic leve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heat Conduction
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy until an object has the same kinetic energy throughout. Thermal conductivity, frequently represented by , is a property that relates the rate of heat loss per unit area of a material to its rate of change of temperature. Essentially, it is a value that accounts for any property of the material that could change the way it conducts heat. Heat spontaneously flows along a temperature gradient (i.e. from a hotter body to a colder body). For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becomin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per metre squared, or pascal-seconds. Viscosity quantifies the internal friction, frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's center line than near its walls. Experiments show that some stress (physics), stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mean Free Path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a result of one or more successive collisions with other particles. Scattering theory Imagine a beam of particles being shot through a target, and consider an infinitesimally thin slab of the target (see the figure). The atoms (or particles) that might stop a beam particle are shown in red. The magnitude of the mean free path depends on the characteristics of the system. Assuming that all the target particles are at rest but only the beam particle is moving, that gives an expression for the mean free path: :\ell = (\sigma n)^, where is the mean free path, is the number of target particles per unit volume, and is the effective cross-sectional area for collision. The area of the slab is , and its volume is . The typical number of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Absolute Zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 °C on the Celsius, Celsius scale, and −459.67 °F on the Fahrenheit scale. The Kelvin and Rankine temperature scales set their zero points at absolute zero by design. This limit can be estimated by extrapolating the ideal gas law to the temperature at which the volume or pressure of a classical gas becomes zero. At absolute zero, there is no thermal motion. However, due to quantum mechanics, quantum effects, the particles still exhibit minimal motion mandated by the Uncertainty principle, Heisenberg uncertainty principle and, for a system of fermions, the Pauli exclusion principle. Even if absolute zero could be achieved, this residual quantum motion would persist. Although absolute zero can be approached, it cannot be reached. Som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Equipartition Theorem
In classical physics, classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energy, energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per Degrees of freedom (physics and chemistry), degree of freedom in translation (physics), translational motion of a molecule should equal that in rotational motion. The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phase Equilibrium
In thermodynamics, the phase rule is a general principle governing multi-component, multi-phase systems in thermodynamic equilibrium. For a system without chemical reactions, it relates the number of freely varying intensive properties () to the number of components (), the number of phases (), and number of ways of performing work on the system (): :F = N + C - P + 1 Examples of intensive properties that count toward are the temperature and pressure. For simple liquids and gases, pressure-volume work is the only type of work, in which case . The rule was derived by American physicist Josiah Willard Gibbs in his landmark paper titled ''On the Equilibrium of Heterogeneous Substances'', published in parts between 1875 and 1878. The number of degrees of freedom (also called the ''variance'') is the number of independent intensive properties, ''i.e.'', the largest number of thermodynamic parameters such as temperature or pressure that can be varied simultaneously and independentl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the Thermodynamic system, system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. It is the subject of study of ''equilibrium chemistry''. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature ( isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz free energy is often used, since explosive reactions by their nature induce pressure changes. It is also frequently used to define fundamental equations of state of pure substances. The concept of free energy was developed by Hermann von Helmholtz, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |