|
Tecartus
Brexucabtagene autoleucel, sold under the brand name Tecartus, is a cell-based gene therapy medication for the treatment of mantle cell lymphoma (MCL) and acute lymphoblastic leukemia (ALL). The most common side effects include serious infections, low blood cell counts and a weakened immune system. The most common side effects for the treatment of ALL include fever, CRS, hypotension, encephalopathy, tachycardias, nausea, chills, headache, fatigue, febrile neutropenia, diarrhea, musculoskeletal pain, hypoxia, rash, edema, tremor, infection with pathogen unspecified, constipation, decreased appetite, and vomiting. Brexucabtagene autoleucel is a chimeric antigen receptor T cell therapy and is the first cell-based gene therapy approved by the U.S. Food and Drug Administration (FDA) for the treatment of mantle cell lymphoma. Brexucabtagene autoleucel was approved for medical use in the United States in July 2020, and in the European Union in December 2020. Medical uses Brexuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
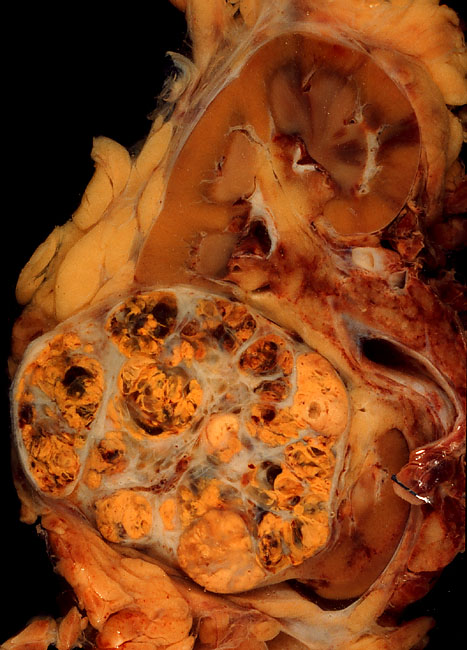
Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) is a type of non-Hodgkin's lymphoma (NHL), comprising about 6% of NHL cases. There are only about 15,000 patients presently in the United States with mantle cell lymphoma. It is named for the mantle zone of the lymph nodes. MCL is a subtype of B-cell lymphoma, due to CD5 positive antigen-naive pregerminal center B-cell within the mantle zone that surrounds normal germinal center follicles. MCL cells generally over-express cyclin D1 due to the t(11:14) translocation, a chromosomal translocation in the DNA. Signs and symptoms At diagnosis, patients typically are in their 60s and present to their physician with advanced disease. About half have B symptoms such as fever, night sweats, or unexplained weight loss (over 10% of body weight). Enlarged lymph nodes (for example, a "bump" on the neck, armpits or groin) or enlargement of the spleen are usually present. Bone marrow, liver and gastrointestinal tract involvement occurs relatively early in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes. Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain. As an acute leukemia, ALL progresses rapidly and is typically fatal within weeks or months if left untreated. In most cases, the cause is unknown. Genetic risk factors may include Down syndrome, Li-Fraumeni syndrome, or neurofibromatosis type 1. Environmental risk factors may include significant radiation exposure or prior chemotherapy. Evidence regarding electromagnetic fields or pesticides is unclear. Some hypothesize that an abnormal immune response to a common infection may be a trigger. The underlying mechanism involves multiple genetic mutations that results in rapid cell division. The excessive immature lymphocytes in the bone marrow interfere with the production of new red blood cells, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DNA was performed in 1980, by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989. The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. It is thought to be able to cure many genetic disorders or treat them over time. Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in phase I.Gene Therapy Cli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DNA was performed in 1980, by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989. The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. It is thought to be able to cure many genetic disorders or treat them over time. Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in phase I.Gene Therapy Cli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilead Sciences
Gilead Sciences, Inc. () is an American biopharmaceutical company headquartered in Foster City, California, that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500. Gilead was founded in 1987 under the name Oligogen by Michael L. Riordan. The original name was a reference to oligomers, small strands of DNA used to target genetic sequences. Gilead held its IPO in 1992, and successfully developed drugs like Tamiflu and Vistide that decade. In the 2000s, Gilead received approval for drugs including Viread and Hepsera, among others. It began evolving from a biotechnology company into a pharmaceutical company, acquiring several subsidiaries, though it still relied heavily on contracting to manufacture its drugs. The company continued its growth in the 2010s, but came under heavy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chimeric Antigen Receptor T Cell
In biology, chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors—are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor. CAR T cell therapy uses T cells engineered with CARs to treat cancer. The premise of CAR T immunotherapy is to modify T cells to recognize cancer cells in order to more effectively target and destroy them. Scientists harvest T cells from people, genetically alter them, then infuse the resulting CAR T cells into patients to attack their tumors. CAR T cells can be derived either from T cells in a patient's own blood (autologously) or from the T cells of another, healthy, donor ( allogeneically). Once isolated from a person, these T cells are genetically engineered to express a specific CAR, which programs t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerated Approval (FDA)
The United States Food and Drug Administration (FDA) initiated the FDA Accelerated Approval Program in 1992 to allow faster approval of drugs for serious conditions that fill an unmet medical need. The faster approval relies on use of surrogate endpoints. Drug approval typically requires clinical trials with endpoints that demonstrate a clinical benefit, such as increased survival for cancer patients. Drugs with accelerated approval can initially be tested in clinical trials that use a surrogate endpoint, or something that is thought to predict clinical benefit. Surrogate endpoints typically require less time, and in the case of a cancer patient, it is much faster to measure a reduction in tumor size, for example, than overall patient survival. Drugs approved under the FDA Accelerated Approval Program still need to be tested in clinical trials using endpoints that demonstrate clinical benefit, and those trials are known as phase 4 confirmatory trials. If the drug later proves unabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer Treatments
Cancer can be treated by surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy (including immunotherapy such as monoclonal antibody therapy) and synthetic lethality, most commonly as a series of separate treatments (e.g. chemotherapy before surgery). The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient (performance status). Cancer genome sequencing helps in determining which cancer the patient exactly has for determining the best therapy for the cancer. A number of experimental cancer treatments are also under development. Under current estimates, two in five people will have cancer at some point in their lifetime. Complete removal of the cancer without damage to the rest of the body (that is, achieving cure with near-zero adverse effects) is the ideal, if rarely achieved, goal of treatment and is often the goal in practice. Sometimes this can be accomplished by sur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |