|
Tantalum Carbide
Tantalum carbides (TaC) form a family of binary compound, binary chemical compounds of tantalum and carbon with the empirical formula TaC''x'', where ''x'' usually varies between 0.4 and 1. They are extremely hardness, hard, brittle, refraction (metallurgy), refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering. Being important cermet materials, tantalum carbides are commercially used in tool bits for cutting applications and are sometimes added to tungsten carbide alloys. The melting points of tantalum carbides was previously estimated to be about 3,880 °C depending on the purity and measurement conditions; this value is among the highest for binary compounds. And only tantalum hafnium carbide was estimated to have a higher melting point of 3,942 °C. However new tests have conclusively proven that TaC actually has a melting point of 3,768 °C and both tantalum hafnium carbid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6, cryolite, and AlF3, aluminium trifluoride. A molten mixture of these solids serves as a high-temperature solvent for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
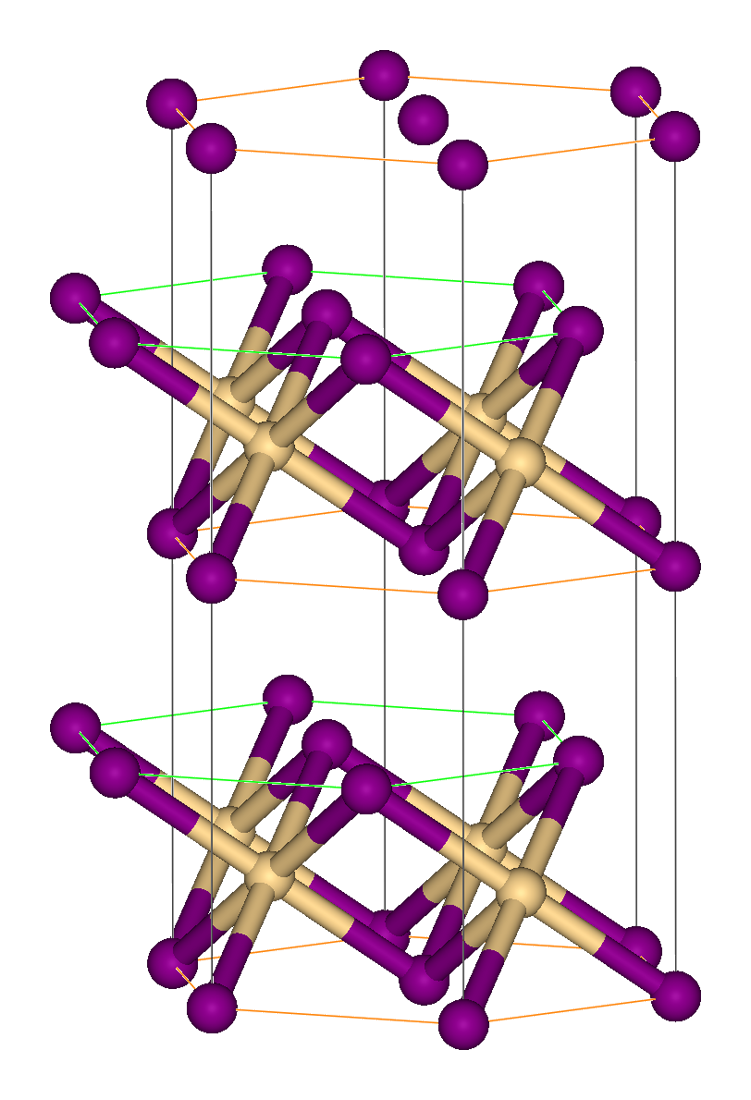
Tantalum Hafnium Carbide
Tantalum hafnium carbide is a refractory chemical compound with a general formula , which can be considered as a solid solution of tantalum carbide and hafnium carbide. Properties Individually, tantalum and hafnium carbide have the highest melting points among the binary compounds, and , respectively, and their "alloy" with a composition Ta4HfC5 has an melting point of . Very few measurements of melting point in tantalum hafnium carbide have been reported, because of the obvious experimental difficulties at extreme temperatures. A 1965 study of the TaC-HfC solid solutions at temperatures 2,225–2,275 °C found a minimum in the vaporization rate and thus maximum in the thermal stability for Ta4HfC5. This rate was comparable to that of tungsten and was weakly dependent on the initial density of the samples, which were sintered from TaC-HfC powder mixtures, also at 2,225–2,275 °C. In a separate study, Ta4HfC5 was found to have the minimum oxidation rate among the Ta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source. The superconductivity phenomenon was discovered in 1911 by Dutch physicist Heike Kamerlingh Onnes. Like ferromagnetism and atomic spectral lines, superconductivity is a phenomenon which can only be explained by quantum mechanics. It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor during its transitions into th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastic Modulus
An elastic modulus (also known as modulus of elasticity) is the unit of measurement of an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it. The elastic modulus of an object is defined as the slope of its stress–strain curve in the elastic deformation region: A stiffer material will have a higher elastic modulus. An elastic modulus has the form: :\delta \ \stackrel\ \frac where stress is the force causing the deformation divided by the area to which the force is applied and strain is the ratio of the change in some parameter caused by the deformation to the original value of the parameter. Since strain is a dimensionless quantity, the units of \delta will be the same as the units of stress. Specifying how stress and strain are to be measured, including directions, allows for many types of elastic moduli to be defined. The three primary ones are: # '' Young's modulus'' (E) describes tensile and compres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-propagating High-temperature Synthesis
Self-propagating high-temperature synthesis (SHS) is a method for producing both inorganic and organic compounds by exothermic combustion reactions in solids of different nature. Reactions can occur between a solid reactant coupled with either a gas, liquid, or other solid. If the reactants, intermediates, and products are all solids, it is known as a solid flame. If the reaction occurs between a solid reactant and a gas phase reactant, it is called infiltration combustion. Since the process occurs at high temperatures, the method is ideally suited for the production of refractory materials including powders, metallic alloys, or ceramics. The modern SHS process was reported and patented in 1971,"''Self-propagated high-temperature synthesis of refractory inorganic compounds''", A.G. Merzhanov, I.P. Borovinskaya. Doklady Akademii Nauk SSSR, Vol. 204, N 2, pp. 366-369, May, 1972 although some SHS-like processes were known previously. Advantages and Disadvantages Self-propagating high- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum Pentoxide
Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula . It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. is an inert material with a high refractive index and low absorption (i.e. colourless), which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant. Preparation Occurrence Tantalum occurs in the minerals tantalite and columbite (columbium being an archaic name for niobium), which occur in pegmatites, an igneous rock formation. Mixtures of columbite and tantalite are called coltan. Tantalite was discovered by Anders Gustaf Ekeberg at Ytterby, Sweden, and Kimoto, Finland. The minerals microlite and pyrochlore contain approximately 70% and 10% Ta, respectively. Refining Tantalum ores often contain significant amounts of niobium, which is itself a valuable metal. As such, both metals are extracted so tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_0468.jpg)