|
Tamejiro Hiyama
Tamejiro Hiyama (born August 24, 1946) is a Japanese organic chemistry, organic chemist. He is best known for his work in developing the Nozaki-Hiyama-Kishi reaction and the Hiyama coupling. He is currently a professor at the Chuo University Research and Development Initiative, and a Professor Emeritus of Kyoto University. Career Hiyama received his Bachelor of Engineering (1969) and Master of Engineering (1971) from Kyoto University. He dropped out of the doctorate track in 1972, and subsequently started working as an assistant for Hitoshi Nozaki at Kyoto University. In 1975, he obtained his doctoral degree, and during 1975-1976 conducted postdoctoral research with Yoshito Kishi at Harvard University. In 1981, he started working at the Sagami Chemical Research Center, and became a principal investigator in 1983, and then chief laboratory manager in 1988. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibaraki, Osaka
is a city in Osaka Prefecture, Japan. It is a suburban city of Osaka City and a part of the Kyoto-Osaka-Kobe metropolitan area. Ibaraki translates to "wild trees" or "thorny trees". The city was incorporated on 1 January 1948. As of February 2017, the city has an estimated population of 280,562 and a population density of 3,580 persons per km2. The total area is 76.52 km2. Transportation Railways *West Japan Railway Company **JR Kyoto Line: Ibaraki Station– JR-Sōjiji Station *Hankyu Railway **Hankyu Kyoto Line: Minami-Ibaraki Station–Ibaraki-shi Station– Sōjiji Station *Osaka Monorail **Main Line: Unobe Station– Minami-Ibaraki Station– Sawaragi Station **Saito Line Handai-byōin-mae Station– Toyokawa Station–Saito-nishi Station Highways * * Education Prefectural public senior high schools: * * * * * * Private junior and senior high schools: * * * * Korea International School, a Korean international junior and senior high school, is in Ibaraki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yoshito Kishi
is a Japanese chemist who is the Morris Loeb Professor of Chemistry at Harvard University. He is known for his contributions to the sciences of organic synthesis and total synthesis. Kishi was born in Nagoya, Japan and attended Nagoya University, where he obtained both his BS and PhD degrees. He was a postdoctoral research fellow at Harvard University where he worked with Robert Burns Woodward. From 1966 through 1974, he was a professor of chemistry at Nagoya University. Since 1974, Kishi has been a professor of chemistry at Harvard University. Kishi's research has focused on the total synthesis of complex natural products. The accomplishments of his research group include the total syntheses of palytoxin, mycolactones, halichondrins, saxitoxin, tetrodotoxin, geldanamycin, batrachotoxin and many others. Kishi has also contributed to the development of new chemical reactions including the Nozaki–Hiyama–Kishi reaction. Recognition *1999 Imperial Prize of the Japan Academ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Halide
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications. Preparation The two main preparatory routes to aryl halides are direct halogenation and via diazonium salts. Direct halogenation In the Friedel-Crafts halogenation, Lewis acids serve as catalysts. Many metal chlorides are used, examples include iron(III) chloride or aluminium chloride. The most important aryl halide, chlorobenzene is produced by this route. Monochlorination of benzene is always accompanied by formation of the dichlorobenzene derivatives. Arenes with electron donating groups react with halogens even in the absence of Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Halide
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl halides. From the perspective of applications, the dominant member of this class of compounds is vinyl chloride, which is produced on the scale of millions of tons per year as a precursor to polyvinyl chloride. Polyvinyl fluoride is another commercial product. Related compounds include 1,1-Dichloroethene, vinylidene chloride and vinylidene fluoride. Synthesis Vinyl chloride is produced by dehydrochlorination of 1,2-dichloroethane. Due to their high utility, many approaches to vinyl halides have been developed, such as: * reactions of vinyl organometallic species with halogens * Takai olefination * Stork-Zhao olefination - a modification of the Wittig reaction * Olefin metathesis Reactions Vinyl bromide and related alkenyl halid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Halide
Allyl halides are organic halides containing an allyl group. Allyl halides include: * Allyl chloride * Allyl bromide * Allyl iodide Allyl iodide (3-iodopropene) is an organic halide used in synthesis of other organic compounds such as ''N''-alkyl- 2-pyrrolidones, sorbic acid esters, 5,5-disubstituted barbituric acids, and organometallic catalysts. Allyl iodide can be synthesi ... * See also * {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-coupling Reaction
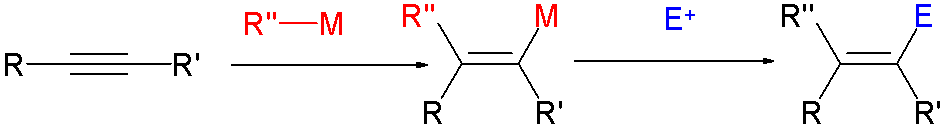
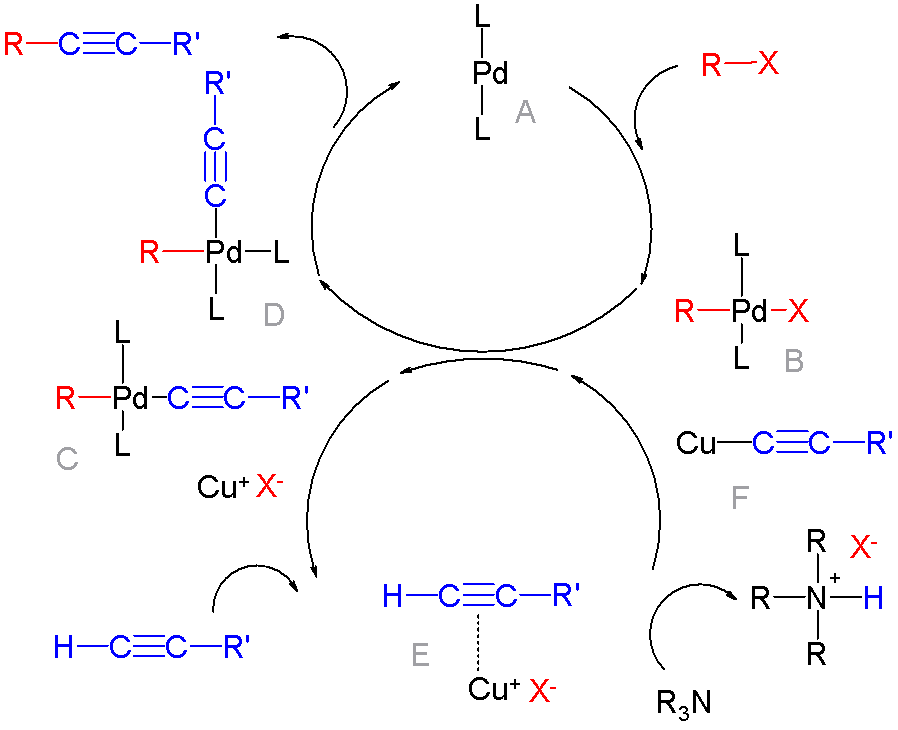
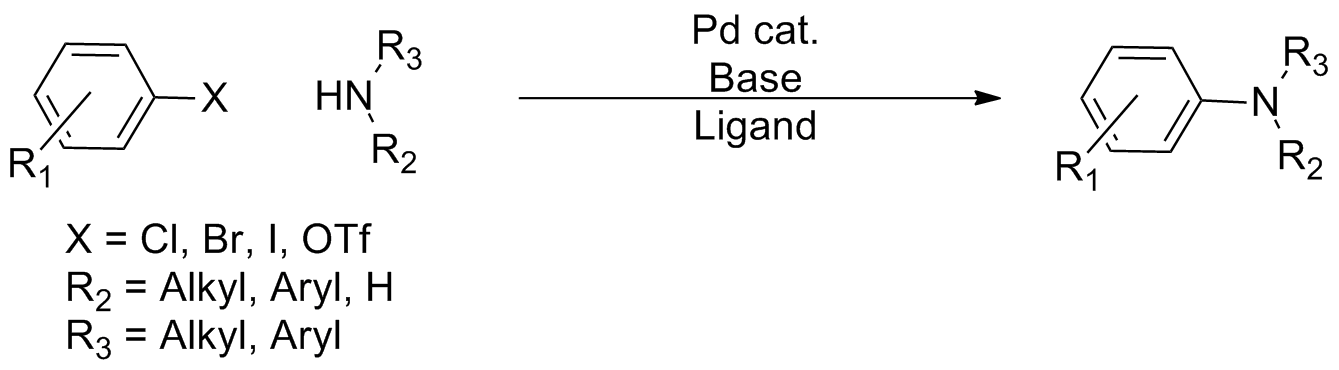
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subsequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal. Chromium metal is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared light. The name of the element is derived from the Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Industrial production of chromium proceeds from chromite ore (mostly FeCr2O4) to produce ferro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buddhist
Buddhism ( , ), also known as Buddha Dharma and Dharmavinaya (), is an Indian religion or philosophical tradition based on teachings attributed to the Buddha. It originated in northern India as a -movement in the 5th century BCE, and gradually spread throughout much of Asia via the Silk Road. It is the world's fourth-largest religion, with over 520 million followers (Buddhists) who comprise seven percent of the global population. The Buddha taught the Middle Way, a path of spiritual development that avoids both extreme asceticism and hedonism. It aims at liberation from clinging and craving to things which are impermanent (), incapable of satisfying ('), and without a lasting essence (), ending the cycle of death and rebirth (). A summary of this path is expressed in the Noble Eightfold Path, a training of the mind with observance of Buddhist ethics and meditation. Other widely observed practices include: monasticism; " taking refuge" in the Buddha, the , and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials in 1904. In recognition of Kipping's achiev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-coupling
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subseq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |