|
TRPV
TRPV is a family of transient receptor potential cation channels (TRP channels) in animals. All TRPVs are highly calcium selective. TRP channels are a large group of ion channels consisting of six protein families, located mostly on the plasma membrane of numerous human and animal cell types, and in some fungi. TRP channels were initially discovered in the ''trp'' mutant strain of the fruit fly ''Drosophila'' that displayed transient elevation of potential in response to light stimuli, and were therefore named "transient receptor potential" channels. The name now refers only to a family of proteins with similar structure and function, not to the mechanism of their activation. Later, TRP channels were found in vertebrates where they are ubiquitously expressed in many cell types and tissues. There are about 28 TRP channels that share some structural similarity to each other. These are grouped into two broad groups: group 1 includes TRPC ( "C" for canonical), TRPV ("V" for vanill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRPV1
The transient receptor potential cation channel subfamily V member 1 (TrpV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the ''TRPV1'' gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 (a chemical irritant receptor) to mediate the detection of noxious environmental stimuli. Function TRPV1 is an element of or mechanism used by the mammalian somatosensory system. It is a nonselective cation channel that may be activated by a wide variety of exogenous and endogenous physical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transient Receptor Potential Channel
Transient receptor potential channels (TRP channels) are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC ( "C" for canonical), TRPV ("V" for vanilloid), TRPVL ("VL" for vanilloid-like), TRPM ("M" for melastatin), TRPS ("S" for soromelastatin), TRPN ("N" for no mechanoreceptor potential C), and TRPA ("A" for ankyrin). Group 2 consists of TRPP ("P" for polycystic) and TRPML ("ML" for mucolipin). Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
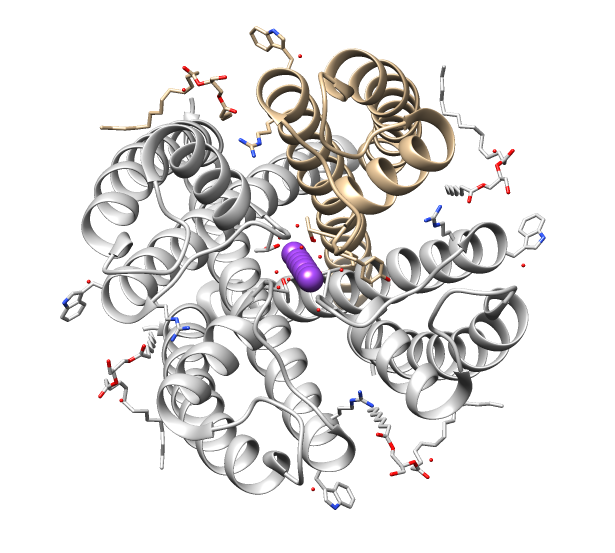
TRPV2
TRPV is a family of transient receptor potential cation channels (TRP channels) in animals. All TRPVs are highly calcium selective. TRP channels are a large group of ion channels consisting of six protein families, located mostly on the plasma membrane of numerous human and animal cell types, and in some fungi. TRP channels were initially discovered in the ''trp'' mutant strain of the fruit fly ''Drosophila'' that displayed transient elevation of potential in response to light stimuli, and were therefore named "transient receptor potential" channels. The name now refers only to a family of proteins with similar structure and function, not to the mechanism of their activation. Later, TRP channels were found in vertebrates where they are ubiquitously expressed in many cell types and tissues. There are about 28 TRP channels that share some structural similarity to each other. These are grouped into two broad groups: group 1 includes TRPC ( "C" for canonical), TRPV ("V" for vanillo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoreceptor
A thermoreceptor is a non-specialised sense receptor, or more accurately the receptive portion of a sensory neuron, that codes absolute and relative changes in temperature, primarily within the innocuous range. In the mammalian peripheral nervous system, warmth receptors are thought to be unmyelinated C-fibres (low conduction velocity), while those responding to cold have both C-fibers and thinly myelinated A delta fibers (faster conduction velocity). The adequate stimulus for a warm receptor is warming, which results in an increase in their action potential discharge rate. Cooling results in a decrease in warm receptor discharge rate. For cold receptors their firing rate increases during cooling and decreases during warming. Some cold receptors also respond with a brief action potential discharge to high temperatures, i.e. typically above 45 °C, and this is known as a paradoxical response to heat. The mechanism responsible for this behavior has not been determined. Loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsaicin
Capsaicin (8-methyl-''N''-vanillyl-6-nonenamide) ( or ) is an active component of chili peppers, which are plants belonging to the genus ''Capsicum''. It is a chemical irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related alkaloids are called capsaicinoids and are produced as secondary metabolites by chili peppers, probably as deterrents against certain mammals and fungi.What Made Chili Peppers So Spicy? Talk of the Nation, 15 August 2008. Pure capsaicin is a , colorless, highly |
Vanilloid
The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids. : A number of vanilloids, most notably capsaicin, bind to the transient receptor potential vanilloid type 1 (TRPV1) receptor, an ion channel which naturally responds to noxious stimuli such as high temperatures and acidic pH. This action is responsible for the burning sensation experienced after eating spicy peppers. Endogenously generated chemicals that trigger the TRPV1 channel of the vanilloids class are referred to as endovanilloids including anandamide, 20-Hydroxyeicosatetraenoic_acid(20-HETE) ,N-Arachidonoyl_dopamine (NADA) and N-oleoyl-dopamine. : Outside the food industry vanilloids such as nonivamide are used commercially in pepper spray formulations. Other vanilloids which act at TRPV1 include resiniferatoxin Resiniferatoxin (RTX) is a natura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperine
Piperine, along with its isomer chavicine, is the alkaloid responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine. Preparation Due to its poor solubility in water, piperine is typically extracted from black pepper by using organic solvents like dichloromethane. The amount of piperine varies from 1–2% in long pepper, to 5–10% in commercial white and black peppers. Piperine can also be prepared by treating a concentrated alcoholic extract of black pepper with an alcoholic solution of potassium hydroxide to remove resin (said to contain chavicine, an isomer of piperine). The solution is decanted from the insoluble residue and left to stand overnight. During this period, the alkaloid slowly crystallizes from the solution. Piperine has been synthesized by the action of piperonoyl chloride on piperidine. Reactions Piperine forms salts only with strong acids. The platinichloride B4·H2PtCl6 forms orange-red needles ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRPM
TRPM is a family of transient receptor potential ion channels (''M'' standing for '' wikt:melastatin''). Functional TRPM channels are believed to form tetramers. The TRPM family consists of eight different channels, TRPM1–TRPM8. Unlike the TRPC and TRPV sub-families, TRPM subunits do not contain N-terminal ankyrin repeat motifs but, rather, contain entire functional proteins in their C-termini. TRPM6 and TRPM7, for example, contain functional α-kinase segments, which are a type of serine/threonine-specific protein kinase. Permeability and activation The relative permeability of calcium and magnesium varies widely among TRPM channels. *TRPM4 and TRPM5 are impermeable to calcium. *TRPM3, TRPM6 and TRPM7 are highly permeable to both calcium and magnesium. The mechanism of activation also varies greatly among TRPM channels. *TRPM2 is activated by ADP-ribose adenosine 5'-diphosphoribose and functions as a sensor of redox status in cells. *TRPM4 and TRPM5 are activated by intra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRPN
TRPN is a member of the transient receptor potential channel family of ion channels, which is a diverse group of proteins thought to be involved in mechanoreception. The TRPN gene was given the name ''no mechanoreceptor potential C'' (''nompC'') when it was first discovered in fruit flies, hence the ''N'' in TRPN. Since its discovery in fruit flies, TRPN homologs have been discovered and characterized in worms, frogs, and zebrafish. Structure A structure of NOMPC was published in 2017, solved using electron cryo-microscopy. X-ray crystallography studies of channel segments cloned from fruit flies and zebrafish have led to the hypothesis that multiple ankyrin repeats at TRPN's N-terminus are involved in the gating of the channel pore. Crystallography studies of TRPY1, a yeast TRP homolog, have shown that aromatic residues conserved across TRP family members, including TRPN, in the sixth transmembrane domain are critical to the gating mechanism as well. Function As a mechanoreceptor, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all parts of the bodies of bilaterally symmetric and triploblastic animals—that is, all multicellular animals except sponges and diploblasts. It is a structure composed of nervous tissue positioned along the rostral (nose end) to caudal (tail end) axis of the body and may have an enlarged section at the rostral end which is a brain. Only arthropods, cephalopods and vertebrates have a true brain (precursor structures exist in onychophorans, gastropods and lancelets). The rest of this article exclusively discusses the vertebrate central nervous system, which is radically distinct from all other animals. Overview In vertebrates, the brain and spinal cord are both enclosed in the meninges. The meninges provide a barrier to chemicals dissolv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |