|
TRIM22
Tripartite motif-containing 22, also known as TRIM22, is a protein which in humans is encoded by the ''TRIM22'' gene. Function The protein encoded by this gene is a member of the tripartite motif (TRIM) family. The TRIM motif includes three zinc-binding domains, a RING, a B-box type 1 and a B-box type 2, and a coiled-coil region. This protein localizes to the cytoplasm and its expression is induced by interferon. TRIM22 is also a target gene of the tumor suppressor protein p53. TRIM22 possesses E3 ubiquitin ligase activity and is able to ubiquitinate itself with the assistance of the E2 enzyme UbcH5B. Furthermore, TRIM22 is located in the nucleus and therefore may function as a nuclear E3 ubiquitin ligase. Clinical significance The protein down-regulates transcription from the HIV-1 long terminal repeat promoter region, suggesting that function of this protein may be to mediate interferon's antiviral effects. Other proteins that function to restrict HIV repli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tripartite Motif Family
The tripartite motif family (TRIM) is a protein family. Function Many TRIM proteins are induced by interferons, which are important component of resistance to pathogens and several TRIM proteins are known to be required for the restriction of infection by lentiviruses. TRIM proteins are involved in pathogen-recognition and by regulation of transcriptional pathways in host defence. Structure The tripartite motif is always present at the N-terminus of the TRIM proteins. The TRIM motif includes the following three domains: * (1) a RING finger domain * (2) one or two B-box zinc finger domains ** when only one B-box is present, it is always a type-2 B-box ** when two B-boxes are present the type-1 B-Box always precedes the type-2 B-Box * (3) coiled coil region The C-terminus of TRIM proteins contain either: * Group 1 proteins: a C-terminal domain selected from the following list: ** NHL and IGFLMN domains, either in association or alone ** PHD domain associated with a bromodomain ** ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RING Finger Domain
In molecular biology, a RING (short for Really Interesting New Gene) finger domain is a protein structural domain of zinc finger type which contains a C3HC4 amino acid motif which binds two zinc cations (seven cysteines and one histidine arranged non-consecutively). This protein domain contains 40 to 60 amino acids. Many proteins containing a RING finger play a key role in the ubiquitination pathway. Zinc fingers Zinc finger (Znf) domains are relatively small protein motifs that bind one or more zinc atoms, and which usually contain multiple finger-like protrusions that make tandem contacts with their target molecule. They bind DNA, RNA, protein and/or lipid substrates. Their binding properties depend on the amino acid sequence of the finger domains and of the linker between fingers, as well as on the higher-order structures and the number of fingers. Znf domains are often found in clusters, where fingers can have different binding specificities. There are many superfamilies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRIM5alpha
Tripartite motif-containing protein 5 also known as RING finger protein 88 is a protein that in humans is encoded by the ''TRIM5'' gene. The alpha isoform of this protein, TRIM5α, is a retrovirus restriction factor, which mediates a species-specific early block to retrovirus infection. TRIM5α is composed of 493 amino acids which is found in the cell (biology), cells of most primates. TRIM5α is an intrinsic immune factor important in the innate immune system, innate immune defense against retroviruses, along with the APOBEC family of proteins, tetherin and TRIM22. Structure TRIM5α belongs to the tripartite motif family, TRIM protein family (TRIM stands for TRIpartite Motif); this family was first identified by Reddy in 1992 as a set of proteins which contain a RING finger domain, RING type zinc finger domain, a B-box zinc binding domain, followed by a coiled coil, coiled-coil region. TRIM5α bears the C-terminus, C-terminal PRY-SPRY or B30.2 domain in addition to the other d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UBE2D2
Ubiquitin-conjugating enzyme E2 D2 is a protein that in humans is encoded by the ''UBE2D2'' gene. Function The modification of proteins with ubiquitin is an important cellular mechanism for targeting abnormal or short-lived proteins for degradation. Ubiquitination involves at least three classes of enzymes: ubiquitin-activating enzymes, or E1s, ubiquitin-conjugating enzymes, or E2s, and ubiquitin-protein ligases, or E3s. This gene encodes a member of the E2 ubiquitin-conjugating enzyme family. This enzyme functions in the ubiquitination of the tumor-suppressor protein p53, which is induced by an E3 ubiquitin-protein ligase. Two alternatively spliced transcript variants have been found for this gene and they encode distinct isoforms. Interactions UBE2D2 has been shown to interact with: * Baculoviral IAP repeat-containing protein 3, * NEDD4, * PJA1, * PJA2, and * UBE3A Ubiquitin-protein ligase E3A (UBE3A) also known as E6AP ubiquitin-protein ligase (E6AP) is an enzyme th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interferon Type I
The type-I interferons (IFN) are cytokines which play essential roles in inflammation, immunoregulation, tumor cells recognition, and T-cell responses. In the human genome, a cluster of thirteen functional IFN genes is located at the 9p21.3 cytoband over approximately 400 kb including coding genes for IFNα (''IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17'' and ''IFNA21''), IFNω (''IFNW1''), IFNɛ (''IFNE''), IFNк (''IFNK'') and IFNβ (''IFNB1''), plus 11 IFN pseudogenes. Interferons bind to interferon receptors. All type I IFNs bind to a specific cell surface receptor complex known as the IFN-α receptor (IFNAR) that consists of IFNAR1 and IFNAR2 chains. Type I IFNs are found in all mammals, and homologous (similar) molecules have been found in birds, reptiles, amphibians and fish species. Sources and functions IFN-α and IFN-β are secreted by many cell types including lymphocytes (NK cells, B-cells and T-cells), macrophages, fib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
APOBEC3G
APOBEC3G (apolipoprotein B mRNA editing enzyme, catalytic subunit 3G) is a human enzyme encoded by the ''APOBEC3G'' gene that belongs to the APOBEC superfamily of proteins. This family of proteins has been suggested to play an important role in innate anti-viral immunity. APOBEC3G belongs to the family of cytidine deaminases that catalyze the deamination of cytidine to uridine in the single stranded DNA substrate. The C-terminal domain of A3G renders catalytic activity, several NMR and crystal structures explain the substrate specificity and catalytic activity. APOBEC3G exerts innate antiretroviral immune activity against retroviruses, most notably HIV, by interfering with proper replication. However, lentiviruses such as HIV have evolved the Viral infectivity factor (Vif) protein in order to counteract this effect. Vif interacts with APOBEC3G and triggers the ubiquitination and degradation of APOBEC3G via the proteasomal pathway. On the other hand, foamy viruses produce an ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Long Terminal Repeat
A long terminal repeat (LTR) is a pair of identical sequences of DNA, several hundred base pairs long, which occur in eukaryotic genomes on either end of a series of genes or pseudogenes that form a retrotransposon or an endogenous retrovirus or a retroviral provirus. All retroviral genomes are flanked by LTRs, while there are some retrotransposons without LTRs. Typically, an element flanked by a pair of LTRs will encode a reverse transcriptase and an integrase, allowing the element to be copied and inserted at a different location of the genome. Copies of such an LTR-flanked element can often be found hundreds or thousands of times in a genome. LTR retrotransposons comprise about 8% of the human genome. The first LTR sequences were found by A.P. Czernilofsky and J. Shine in 1977 and 1980. Transcription The LTR-flanked sequences are partially transcribed into an RNA intermediate, followed by reverse transcription into complementary DNA (cDNA) and ultimately dsDNA (double-stra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subtypes Of HIV
The subtypes of HIV include two major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is related to viruses found in chimpanzees and gorillas living in western Africa, while HIV-2 viruses are related to viruses found in the sooty mangabey, a vulnerable West African primate. HIV-1 viruses can be further divided into groups M, N, O and P. The HIV-1 group M viruses predominate and are responsible for the AIDS pandemic. Group M can be further subdivided into subtypes based on genetic sequence data. Some of the subtypes are known to be more virulent or are resistant to different medications. Likewise, HIV-2 viruses are thought to be less virulent and transmissible than HIV-1 M group viruses, although HIV-2 is also known to cause AIDS. One of the obstacles to treatment of the human immunodeficiency virus (HIV) is its high genetic variability. Major types HIV-1 HIV-1 is the most common and pathogenic strain of the virus. Over 2 million such infections occur annually. Scientists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
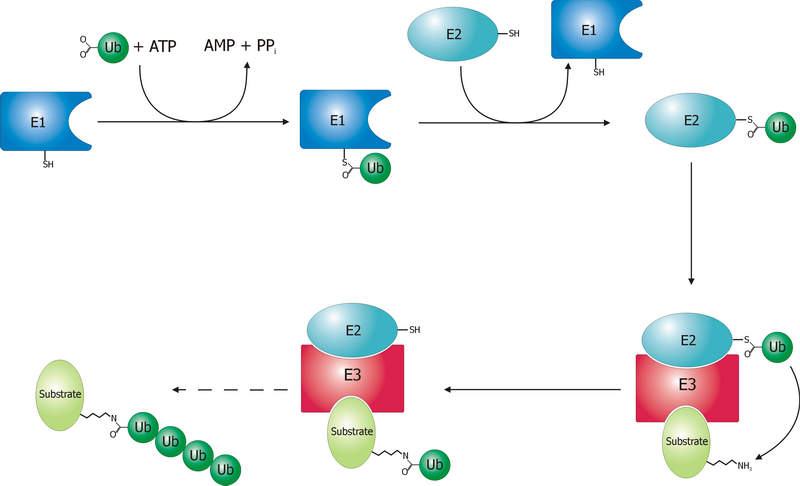
Ubiquitin Ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another thing (the substrate) by some mechanism. The ubiquitin, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or, alternatively, ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |