|
Tungsten(VI) Fluoride
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula W F6. It is a toxic, corrosive, colorless gas, with a density of about (roughly 11 times heavier than air). It is one of the densest known gases under standard conditions. WF6 ls commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides. Properties The WF6 molecule is octahedral with the symmetry point group of Oh. The W–F bond distances are . p. 4-93. Between , tungsten hexafluoride condenses into a pale yellow liquid having the density of at . At it freezes into a white solid having a cubic crystalline structure, the lattice constant of 628 pm and calculated density . At this structure transforms into an orthorhombic solid with the lattice constants of , , and , and the density of . In this pha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Hexachloride
Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known. As a d0 ion, W(VI) forms diamagnetic derivatives. The hexachloride is octahedral with equivalent W–Cl distances of 2.24–2.26 Å. Preparation Tungsten hexachloride can be prepared by chlorinating tungsten metal in a sealed tube at 600 °C: : W + 3 Cl2 → WCl6 Properties and Reactions Tungsten (VI) chloride is a blue-black solid at room temperature. At lower temperatures, it becomes wine-red in color. A red form of the compound can be made by rapidly condensing its vapor, which reverts to the blue-black form on gentle heating. It is readily hydrolyzed, even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Common Logarithm
In mathematics, the common logarithm is the logarithm with base 10. It is also known as the decadic logarithm and as the decimal logarithm, named after its base, or Briggsian logarithm, after Henry Briggs, an English mathematician who pioneered its use, as well as standard logarithm. Historically, it was known as ''logarithmus decimalis'' or ''logarithmus decadis''. It is indicated by , , or sometimes with a capital (however, this notation is ambiguous, since it can also mean the complex natural logarithmic multi-valued function). On calculators, it is printed as "log", but mathematicians usually mean natural logarithm (logarithm with base e ≈ 2.71828) rather than common logarithm when they write "log". To mitigate this ambiguity, the ISO 80000 specification recommends that should be written , and should be . Before the early 1970s, handheld electronic calculators were not available, and mechanical calculators capable of multiplication were bulky, expensive and not widely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents. Structure and bonding The structure of diborane has D2h symmetry. Four hydrides are terminal, while two bridge between the boron centers. The lengths of the B–Hbridge bonds and the B–Hterminal bonds are 1.33 and 1.19 Å respectively. This difference in bond lengths reflects the difference in their strengths, the B–Hbridge bonds being relatively weaker. The weakness of the B–Hbridge compared to B–Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ≈2100 and 2500 cm−1 respectively. The model determined by molecular orbital theory describes the bonds between boron and the termina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germane
Germane is the chemical compound with the formula Ge H4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride. Occurrence Germane has been detected in the atmosphere of Jupiter. Synthesis Germane is typically prepared by reduction of germanium oxides, notably germanates, with hydride reagents such as sodium borohydride, potassium borohydride, lithium borohydride, lithium aluminium hydride, sodium aluminium hydride. The reaction with borohydrides is catalyzed by various acids and can be carried out in either aqueous or organic solvent. On laboratory scale, germane can be prepared by the reaction of Ge(IV) compounds with these hydride reagents. A typical synthesis involved the reaction of potassium germanate with sodium borohydride. :NaHGeO3 + KBH4 + H2O → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : Mg2Si + 4 HCl -> 2 MgCl2 + SiH4 It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : Si + 3 HCl -> HSiCl3 + H2 The trichlorosilane is then converted to a mixture of silane and silicon tetrachloride: : 4 HS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
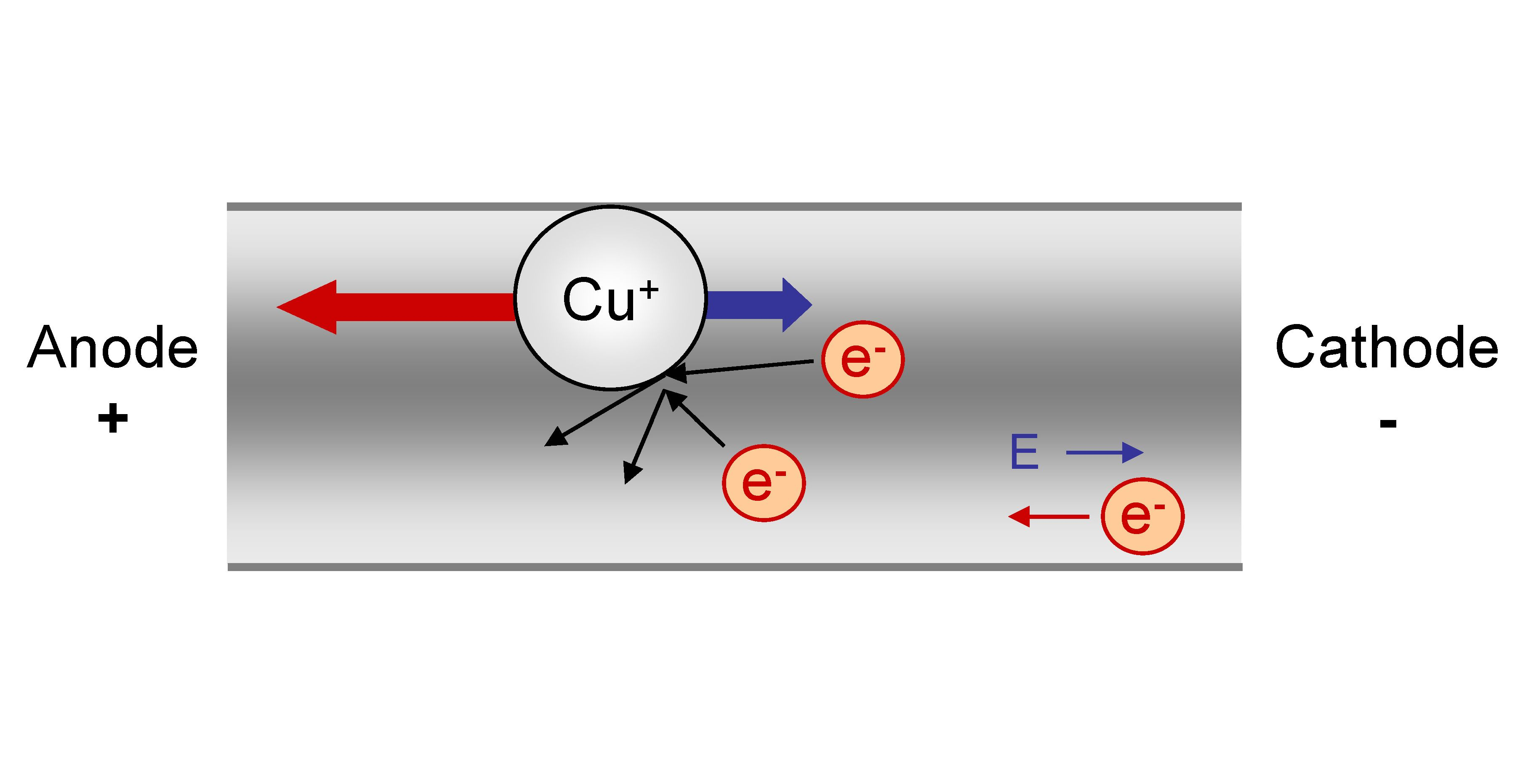
Electromigration
Electromigration is the transport of material caused by the gradual movement of the ions in a conductor due to the momentum transfer between conducting electrons and diffusing metal atoms. The effect is important in applications where high direct current densities are used, such as in microelectronics and related structures. As the structure size in electronics such as integrated circuits (ICs) decreases, the practical significance of this effect increases. History The phenomenon of electromigration has been known for over 100 years, having been discovered by the French scientist Gerardin. The topic first became of practical interest during the late 1960s when packaged ICs first appeared. The earliest commercially available ICs failed in a mere three weeks of use from runaway electromigration, which led to a major industry effort to correct this problem. The first observation of electromigration in thin films was made by I. Blech.I. Blech: ''Electromigration in Thin Aluminum Fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discoveri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Hexachloride
Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known. As a d0 ion, W(VI) forms diamagnetic derivatives. The hexachloride is octahedral with equivalent W–Cl distances of 2.24–2.26 Å. Preparation Tungsten hexachloride can be prepared by chlorinating tungsten metal in a sealed tube at 600 °C: : W + 3 Cl2 → WCl6 Properties and Reactions Tungsten (VI) chloride is a blue-black solid at room temperature. At lower temperatures, it becomes wine-red in color. A red form of the compound can be made by rapidly condensing its vapor, which reverts to the blue-black form on gentle heating. It is readily hydrolyzed, even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Trioxide
Tungsten(VI) oxide, also known as tungsten trioxide is a chemical compound of oxygen and the transition metal tungsten, with formula WO3. The compound is also called tungstic anhydride, reflecting its relation to tungstic acid . It is a light yellow crystalline solid. Tungsten(VI) oxide occurs naturally in the form of hydrates, which include minerals: tungstite WO3·H2O, meymacite WO3·2H2O and hydrotungstite (of the same composition as meymacite, however sometimes written as H2WO4). These minerals are rare to very rare secondary tungsten minerals. History In 1841, a chemist named Robert Oxland gave the first procedures for preparing tungsten trioxide and sodium tungstate. He was granted patents for his work soon after, and is considered to be the founder of systematic tungsten chemistry. Structure and properties The crystal structure of tungsten trioxide is temperature dependent. It is tetragonal at temperatures above 740 °C, orthorhombic from 330 to 740 °C, monoclini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek (bromos) meaning "stench", referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a native element in nature but it occurs in colourless soluble crystalline mineral halide salts, analogous to table salt. In fact, bromine and all the halogens are so reactive that they form bonds in pairs—never in single atoms. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its accumulation in the oceans. Commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |