|
Tufts Center For The Study Of Drug Development
The Tufts Center for the Study of Drug Development is an independent, academic, non-profit research center at Tufts University in Boston, dedicated to researching drug development. It was established in 1976 by American physician Louis Lasagna. The Center develops and publishes information to help researchers, regulators, and policy makers in areas related to the pharmaceutical and biotechnology industries. In any given year, approximately 55% of Tufts CSDD's operating expenses are supported by grants from the private sector and 45% from the public sector. Research The Center studies trends in the pharmaceutical industry, maintaining databases pertaining to investigational new drugs, approved drugs, biopharmaceuticals, fast-tracked drugs, and orphan drugs. The Center provides this information with the aim to improve the efficiency of drug development, foster innovation, and increase patient access to medicines. Drug development costs The center has published numerous studies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Louis Lasagna
Louis Cesare Lasagna (February 22, 1923 – August 6, 2003) was an American physician and professor of medicine, known for his revision of the Hippocratic Oath. Early life and education Lasagna was an internationally recognized and respected expert in clinical pharmacology. Born in Queens, New York in 1923, Lasagna was raised in New Brunswick, New Jersey, by his Italian immigrant parents, and graduated from New Brunswick High School. He graduated from Rutgers University in 1943 and earned his medical degree from Columbia University in 1947. During his time at Rutgers University, he joined Kappa Sigma Fraternity (Gamma-Upsilon). After completing a clinical research fellowship in anesthesia at Harvard Medical School, Lasagna joined the faculty of Johns Hopkins University in 1954, where he established the first ever clinical pharmacology department. Lasagna taught medicine and pharmacology at Johns Hopkins until 1970, when he accepted the position as the first chairman of the D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
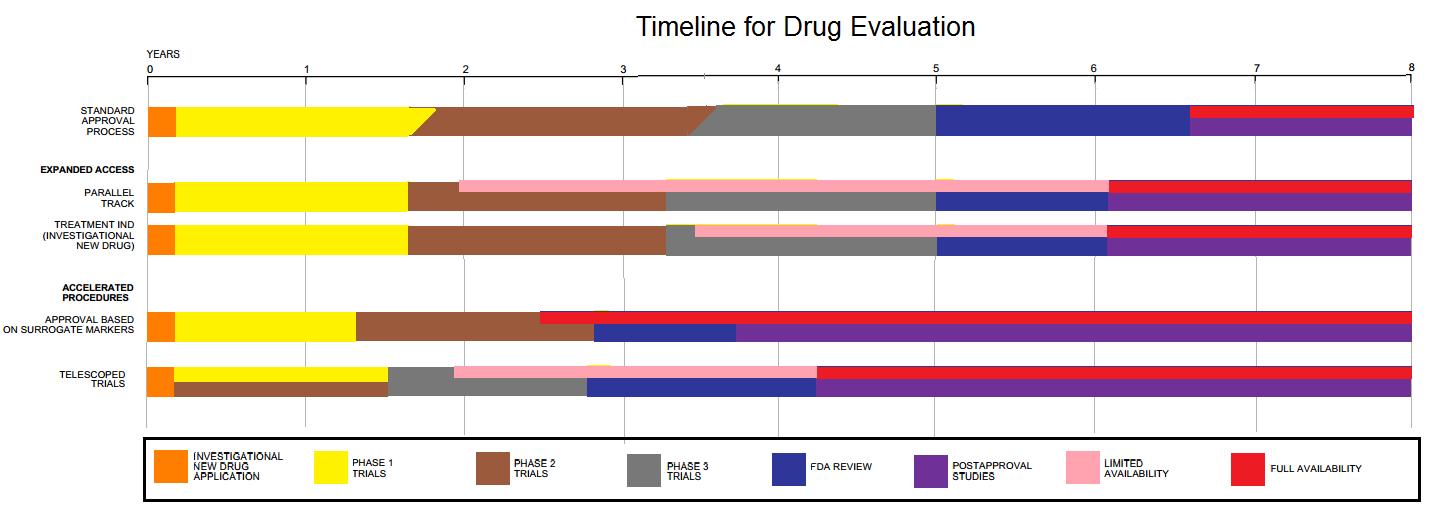
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tufts University
Tufts University is a private research university on the border of Medford and Somerville, Massachusetts. It was founded in 1852 as Tufts College by Christian universalists who sought to provide a nonsectarian institution of higher learning. Tufts remained a small New England liberal arts college until the 1970s, when it transformed into a large research university offering several doctorates;Its corporate name is still "The Trustees of Tufts College" it is classified as a "Research I university", denoting the highest level of research activity. Tufts is a member of the Association of American Universities, a selective group of 64 leading research universities in North America. The university is known for its internationalism, study abroad programs, and promoting active citizenship and public service across all disciplines. Tufts offers over 90 undergraduate and 160 graduate programs across ten schools in the greater Boston area and Talloires, France. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boston
Boston (), officially the City of Boston, is the state capital and most populous city of the Commonwealth of Massachusetts, as well as the cultural and financial center of the New England region of the United States. It is the 24th- most populous city in the country. The city boundaries encompass an area of about and a population of 675,647 as of 2020. It is the seat of Suffolk County (although the county government was disbanded on July 1, 1999). The city is the economic and cultural anchor of a substantially larger metropolitan area known as Greater Boston, a metropolitan statistical area (MSA) home to a census-estimated 4.8 million people in 2016 and ranking as the tenth-largest MSA in the country. A broader combined statistical area (CSA), generally corresponding to the commuting area and including Providence, Rhode Island, is home to approximately 8.2 million people, making it the sixth most populous in the United States. Boston is one of the oldest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopharmaceutical
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine. Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. Some regulatory agencies use the terms ''biological ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast Track is one of five Food and Drug Administration (FDA) approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and Regenerative Medicine Advanced Therapy. Fast Track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Drug
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |