|
Tris(trimethylsilyl)germanium Hydride
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It is extensively used in biochemistry and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary amine and thus undergoes the reactions associated with typical amines, e.g. condensations with aldehydes. Tris also complexes with metal ions in solution. In medicine, tromethamine is occasionally used as a drug, given in intensive care for its properties as a buffer for the treatment of severe metabolic acidosis in specific circumstances. Some medications are formulated as the "tromethamine salt" including Hemabate (carboprost as trometamol salt), and "ketorolac trometamol". Buffering features The conjugate acid of tris has a p''K''a of 8.07 at 25 °C, which implies that the buffer has an effective ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Chloride Electrode
A silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For environmental reasons it has widely replaced the saturated calomel electrode. For example, it is usually the internal reference electrode in pH meters and it is often used as reference in reduction potential measurements. As an example of the latter, the silver chloride electrode is the most commonly used reference electrode for testing cathodic protection corrosion control systems in sea water environments. The electrode functions as a reversible redox electrode and the equilibrium is between the solid (s) silver metal (Ag(s)) and its solid salt—silver chloride (AgCl(s), also called silver(I) chloride) in a chloride solution of a given concentration. In electrochemical cell notation, the silver chloride electrode is written as, ''e.g.'', for an electrolyte solution of KCl 3 M: : \ , \ \ , \ KCl \ (3M) The corresponding half-reactions can be presented as follows ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MES (buffer)
MES is the common name for the compound 2-(''N''-morpholino)ethanesulfonic acid. Its chemical structure contains a morpholine ring. It has a molecular weight of 195.2 and the chemical formula is C6H13NO4S. Synonyms include: 2-morpholinoethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; 2-(N-morpholino)ethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; MES; MES hydrate; and morpholine-4-ethanesulfonic acid hydrate. MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one. Applications MES is used as a buffering agent in biology and biochemistry. It has p''K''a value of 6.15 at 20 °C. The pH (and p''K''a at ionic strength I≠0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators. MES is highly soluble in water. The melting point is approx. 300 °C. MES was developed as one of Good's buffers in the 1960s. These buffers were develop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HEPES
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a zwitterionic sulfonic acid buffering agent; one of the twenty Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration (produced by aerobic respiration) when compared to bicarbonate buffers, which are also commonly used in cell culture. Lepe-Zuniga ''et al.'' reported an unwanted photochemical process wherein HEPES when exposed to ambient light produces hydrogen peroxide, which is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep HEPES-containing solutions in darkness as much as possible to prevent oxidation. HEPES has the following characteristics: * p''K''a1 (25 °C) = 3 * p''K''a2 (25 °C) = 7.5 * Useful pH range = 2.5 to 3.5 or 6.8 to 8.2 HEPES has negligible metal ion binding, making it a good choice as a buffer for enzymes which might be inhibited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOPS
MOPS (3-(''N''-morpholino)propanesulfonic acid) is a buffer introduced in the 1960s, one of the twenty Good's buffers. It is a structural analog to MES, and like MES, its structure contains a morpholine ring. HEPES is a similar pH buffering compound that contains a piperazine ring. With a p''K''a of 7.20, MOPS is an excellent buffer for many biological systems at near-neutral pH. Applications MOPS is frequently used as a buffering agent in biology and biochemistry. It has been tested and recommended for polyacrylamide gel electrophoresis. Usage above 20 mM in mammalian cell culture work is not recommended. MOPS buffer solutions become discolored (yellow) over time, but reportedly slight discoloration does not significantly affect the buffering characteristics. See also *CAPS * HEPPS *Tris Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−). Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs. Nomenclature Because it has long been known and widely used, the salt has many different names such as baking soda, bread soda, cooking soda, and bicarbonate of soda and can often be found near baking powder in stores. The term ''baking soda'' is more common in the United States, while ''bicarbonate of soda'' is more common in Australia, United Kingdom and Ireland. and in many northern/central European countries it is called ''Na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfizer–BioNTech COVID-19 Vaccine
The Pfizer–BioNTech COVID-19 vaccine ( INN: tozinameran), sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech. For its development, BioNTech collaborated with American company Pfizer to carry out clinical trials, logistics, and manufacturing. It is authorized for use in people aged five years and older in some jurisdictions, twelve years and older in some jurisdictions, and for people sixteen years and older in other jurisdictions, to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It is composed of nucleoside-modified mRNA (modRNA) encoding a mutated form of the full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. Initial advice indicated that vaccination required two doses given 21 days apart, but the interval was later extended to up to 42 days in the US, and up to four months i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moderna COVID-19 Vaccine
The Moderna COVID19 vaccine (INN: elasomeran), sold under the brand name Spikevax, is a COVID-19 vaccine developed by American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). Depending on the jurisdiction, it is authorized for use in people aged six months, twelve years, or eighteen years and older. It provides protection against COVID-19 which is caused by infection by the SARS-CoV-2 virus. It is designed to be administered as two or three 0.5 mL doses given by intramuscular injection at an interval of at least 28 days apart. It is an mRNA vaccine composed of nucleoside-modified mRNA (modRNA) encoding a spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. It is authorized for use at some level in many countries. In August and September 2022, bivalent versions of the vaccine (Moderna COVID-19 Vaccine, Bivalent) containing elasome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Standard
A primary standard in metrology is a standard that is sufficiently accurate such that it is not calibrated by or subordinate to other standards. Primary standards are defined via other quantities like length, mass and time. Primary standards are used to calibrate other standards referred to as working standards.Skoog, Douglas A., Donald M. West and F. James Holler. "Fundamentals of Analytical Chemistry 8th ed." Harcourt Brace College Publishers. 1995 ''Holt Science and Technology: Physical Science''. Ed. Rinehart and Winston, Inc. Holt. Holt McDougal (July 2000). . See Hierarchy of Standards. In chemistry Standards are used in analytical chemistry. Here, a primary standard is typically a reagent which can be weighed easily, and which is so pure that its weight is truly representative of the number of moles of substance contained. Features of a primary standard include: # High purity # Stability (low reactivity) # Low hygroscopicity (to minimize weight changes due to humidity) # H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
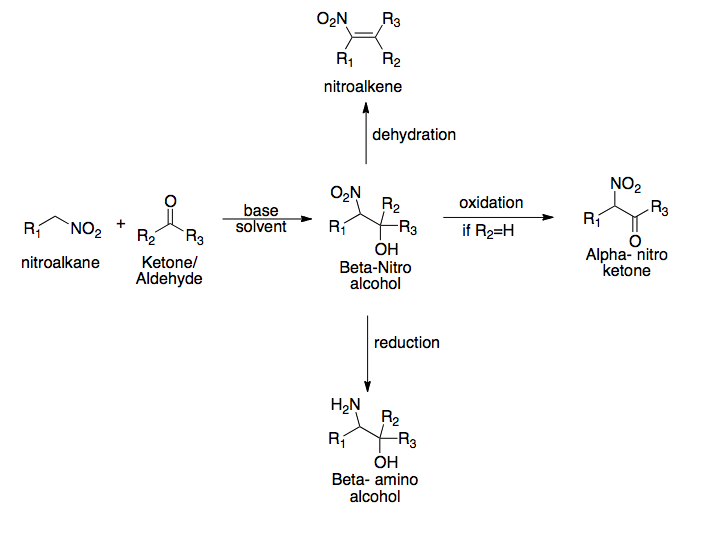
Henry Reaction
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction (nitroalkane, aldehyde, and alcohol). It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_E.jpg)