|
Tissue Inhibitor Of Metalloproteinase
Tissue inhibitors of metalloproteinases (TIMPs) are specific endogenous protease inhibitors to the matrix metalloproteinases. There are four TIMPs; ''TIMP1'', ''TIMP2'', ''TIMP3'' and ''TIMP4''. TIMP3 has been observed progressively downregulated in Human papillomavirus-positive neoplastic keratinocytes derived from uterine cervical preneoplastic lesions at different levels of malignancy. For this reason, TIMP3 is likely to be associated with tumorigenesis and may be a potential prognostic marker for uterine cervical preneoplastic lesions progression. Overall, all MMPs are inhibited by TIMPs once they are activated but the gelatinases (MMP-2 and MMP-9 Matrix metallopeptidase 9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extracel ...) can form complexes with TIMPs when the enzymes are in the latent form. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Metalloproteinase
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily. Collectively, these enzymes are capable of degrading all kinds of extracellular matrix proteins, but also can process a number of bioactive molecules. They are known to be involved in the cleavage of cell surface receptors, the release of apoptotic ligands (such as the FAS ligand), and chemokine/cytokine inactivation. MMPs are also thought to play a major role in cell behaviors such as cell proliferation, migration (adhesion/dispersion), differentiation, angiogenesis, apoptosis, and host defense. They were first described in vertebrates (1962), including humans, but have since been found in invertebrates and plants. They are distinguished from other endopeptida ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TIMP1
TIMP metallopeptidase inhibitor 1, also known as TIMP1, a tissue inhibitor of metalloproteinases, is a glycoprotein with a molecular weight of 28 kDa. TIMP1 is expressed from several tissues of organisms. This protein is a member of the TIMP family. The glycoprotein is a natural inhibitor of the matrix metalloproteinases (MMPs), a group of peptidases involved in degradation of the extracellular matrix. In addition to its inhibitory role against most of the known MMPs, the encoded protein is able to promote cell proliferation in a wide range of cell types, and may also have an anti-apoptotic function. Function TIMP1 is an inhibitory molecule that regulates matrix metalloproteinases (MMPs), and disintegrin-metalloproteinases (ADAMs and ADAMTSs). In regulating MMPs, TIMP1 plays a crucial role in extracellular matrix (ECM) composition, wound healing, and pregnancy. The dysregulated activity of TIMP1 has been implicated in cancer. In pregnancy, TIMP1 plays a regulatory role in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TIMP2
Tissue inhibitor of metalloproteinases 2 (TIMP2) is a gene and a corresponding protein. The gene is a member of the TIMP gene family. The protein is thought to be a metastasis suppressor. Function The proteins encoded by this gene family are natural inhibitors of the matrix metalloproteinases (MMP), a group of peptidases involved in degradation of the extracellular matrix. In addition to an inhibitory role against metalloproteinases, the encoded protein has a unique role among TIMP family members in its ability to directly suppress the proliferation of endothelial cells. As a result, the encoded protein may be critical to the maintenance of tissue homeostasis by suppressing the proliferation of quiescent tissues in response to angiogenic factors, and by inhibiting protease activity in tissues undergoing remodelling of the extracellular matrix. TIMP2 functions as both an MMP inhibitor and an activator. TIMPs inhibit active MMPs, but different TIMPs inhibit different MMPs better ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TIMP3
Metalloproteinase inhibitor 3 is a protein that in humans is encoded by the ''TIMP3'' gene. This gene belongs to the tissue inhibitor of metalloproteinases gene family. The proteins encoded by this gene family are inhibitors of the matrix metalloproteinases, a group of peptidases involved in degradation of the extracellular matrix (ECM). Expression of this gene is induced in response to mitogenic stimulation and this netrin domain-containing protein is localized to the ECM. Mutations in this gene have been associated with the autosomal dominant disorder Sorsby's fundus dystrophy. See also * TIMP1, TIMP2, TIMP4 References Further reading * * * * * * * * * * * * * * * * * * * External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibitor ... online database for pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TIMP4
Metalloproteinase inhibitor 4 is an enzyme that in humans is encoded by the ''TIMP4'' gene. This gene belongs to the tissue inhibitor of metalloproteinases gene family. The proteins encoded by this gene family are inhibitors of the matrix metalloproteinases, a group of peptidases involved in degradation of the extracellular matrix. The secreted, netrin domain-containing protein encoded by this gene is involved in regulation of platelet aggregation and recruitment and may play role in hormonal regulation and endometrial tissue remodeling. Interactions TIMP4 has been shown to interact with MMP2. See also * TIMP1, TIMP2, TIMP3 References Further reading * * * * * * * * * * * * * * * * * * {{refend External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibit ... onli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
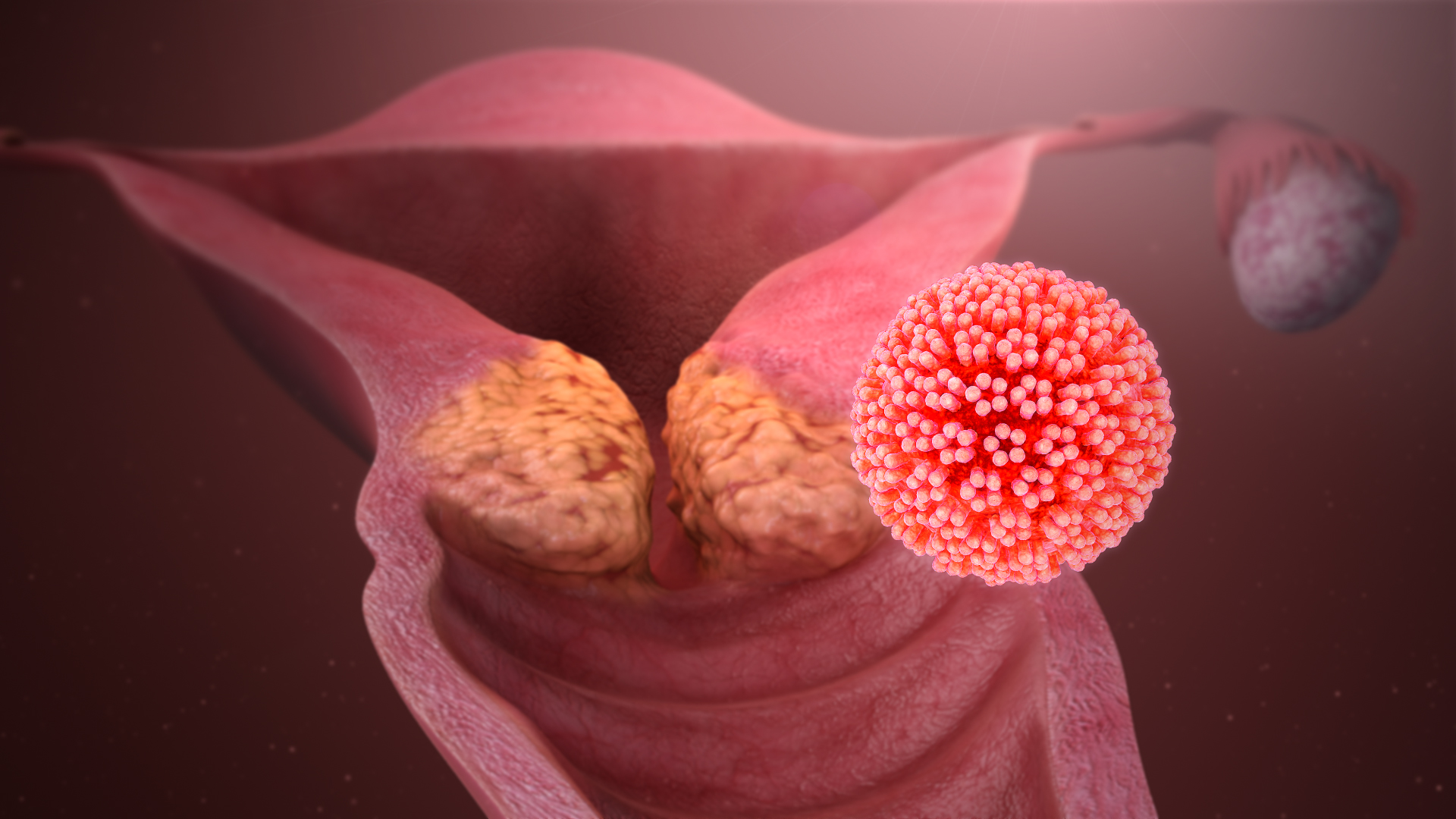
Human Papillomavirus
Human papillomavirus infection (HPV infection) is caused by a DNA virus from the ''Papillomaviridae'' family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. In some cases, an HPV infection persists and results in either warts or precancerous lesions. These lesions, depending on the site affected, increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat. Nearly all cervical cancer is due to HPV and two strains – HPV16 and HPV18 – account for 70% of cases. HPV16 is responsible for almost 90% of HPV-positive oropharyngeal cancers. Between 60% and 90% of the other cancers listed above are also linked to HPV. HPV6 and HPV11 are common causes of genital warts and laryngeal papillomatosis. An HPV infection is caused by ''human papillomavirus'', a DNA virus from the papillomavirus family. Over 170 types have been described. An individual can become infected with more than one type of HPV, and the dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neoplasm
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists in growing abnormally, even if the original trigger is removed. This abnormal growth usually forms a mass, when it may be called a tumor. ICD-10 classifies neoplasms into four main groups: benign neoplasms, in situ neoplasms, malignant neoplasms, and neoplasms of uncertain or unknown behavior. Malignant neoplasms are also simply known as cancers and are the focus of oncology. Prior to the abnormal growth of tissue, as neoplasia, cells often undergo an abnormal pattern of growth, such as metaplasia or dysplasia. However, metaplasia or dysplasia does not always progress to neoplasia and can occur in other conditions as well. The word is from Ancient Greek 'new' and 'formation, creation'. Types A neoplasm can be benign, potentially ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cervical Intraepithelial Neoplasia
Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the abnormal growth of cells on the surface of the cervix that could potentially lead to cervical cancer. More specifically, CIN refers to the potentially precancerous transformation of cells of the cervix. CIN most commonly occurs at the squamocolumnar junction of the cervix, a transitional area between the squamous epithelium of the vagina and the columnar epithelium of the endocervix. It can also occur in vaginal walls and vulvar epithelium. CIN is graded on a 1–3 scale, with 3 being the most abnormal (see classification section below). Human papillomavirus (HPV) infection is necessary for the development of CIN, but not all with this infection develop cervical cancer. Many women with HPV infection never develop CIN or cervical cancer. Typically, HPV resolves on its own. However, those with an HPV infection that lasts more than one or two years have a higher risk of developing a higher grade of CIN. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Metalloproteinase
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily. Collectively, these enzymes are capable of degrading all kinds of extracellular matrix proteins, but also can process a number of bioactive molecules. They are known to be involved in the cleavage of cell surface receptors, the release of apoptotic ligands (such as the FAS ligand), and chemokine/cytokine inactivation. MMPs are also thought to play a major role in cell behaviors such as cell proliferation, migration (adhesion/dispersion), differentiation, angiogenesis, apoptosis, and host defense. They were first described in vertebrates (1962), including humans, but have since been found in invertebrates and plants. They are distinguished from other endopeptida ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelatinase
Gelatinases are enzymes capable of degrading gelatin. Gelatinases are expressed in several bacteria including ''Pseudomonas aeruginosa'' and ''Serratia marcescens''. In humans, the gelatinases are matrix metalloproteinases MMP2 and MMP9 Matrix metallopeptidase 9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extrace .... References EC 3.4.24 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MMP2
72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the ''MMP2'' gene. The ''MMP2'' gene is located on chromosome 16 at position 12.2. Function Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix (ECM) in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. This gene encodes an enzyme which degrades type IV collagen, the major structural component of basement membranes. The enzyme plays a role in endometrial menstrual breakdown, regulation of vascularization and the inflammatory response. Activation Activation of MMP-2 requires proteolytic processing. A complex of membrane type 1 MMP (MT1-MMP/MMP14) and tissue inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MMP9
Matrix metallopeptidase 9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extracellular matrix. In humans the ''MMP9'' gene encodes for a signal peptide, a propeptide, a catalytic domain with inserted three repeats of fibronectin type II domain followed by a C-terminal hemopexin-like domain. Function Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis, bone development, wound healing, cell migration, learning and memory, as well as in pathological processes, such as arthritis, intracerebral hemorrhage, and metastasis. Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |