|
Thermoplastic Polyurethanes
Thermoplastic polyurethane (TPU) is any of a class of polyurethane plastics with many properties, including elasticity, transparency, and resistance to oil, grease, and abrasion. Technically, they are thermoplastic elastomers consisting of linear segmented block copolymers composed of hard and soft segments. Chemistry TPU is a block copolymer consisting of alternating sequences of hard and soft segments or domains formed by the reaction of (1) diisocyanates with short-chain diols (so-called chain extenders) and (2) diisocyanates with long-chain diols. By varying the ratio, structure and/or molecular weight of the reaction compounds, an enormous variety of different TPU can be produced. This allows urethane chemists to fine-tune the polymer's structure to the desired final properties of the material. Morphology A TPU resin consists of linear polymeric chains in block-structures. Such chains contain low polarity segments which are rather long (called soft segments), alternati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many List of polyurethane applications, different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, Potting (electronics), electrical potting compounds, and fibers such as spandex and Polyurethane laminate, PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016. A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as Copolymer#Alternating copolymers, alternating copolymers. Both the isocy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3D Printing
3D printing or additive manufacturing is the Manufacturing, construction of a three-dimensional object from a computer-aided design, CAD model or a digital 3D modeling, 3D model. It can be done in a variety of processes in which material is deposited, joined or solidified under Computer Numerical Control, computer control, with material being added together (such as plastics, liquids or powder grains being fused), typically layer by layer. In the 1980s, 3D printing techniques were considered suitable only for the production of functional or aesthetic prototypes, and a more appropriate term for it at the time was rapid prototyping. , the precision, repeatability, and material range of 3D printing have increased to the point that some 3D printing processes are considered viable as an industrial-production technology, whereby the term ''additive manufacturing'' can be used synonymously with ''3D printing''. One of the key advantages of 3D printing is the ability to produce very ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA). Structure Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Polycarbonates received their name because they are polymers containing carbonate groups (−O−(C=O)−O−). A balance of useful features, including temperature resistance, impact resistance and o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ECHA
The European Chemicals Agency (ECHA; ) is an agency of the European Union which manages the technical and administrative aspects of the implementation of the European Union regulation called Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). ECHA is the driving force among regulatory authorities in implementing the EU's chemicals legislation. ECHA has to ascertain that companies comply with the legislation, advances the safe use of chemicals, provides information on chemicals and addresses chemicals of concern. It is located in Helsinki, Finland. ECHA is an independent and mature regulatory agency established by REACH. It is not a subsidiary entity of the European Commission. The agency, currently headed by Acting Executive Director Shay O’Malley, started working on 1 June 2007. Establishment The ECHA was created by European Union regulation dating from 18 December 2006 to manage the then-new legislation to regulate the manufacture and use of chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substance Of Very High Concern
A substance of very high concern (SVHC) is a chemical substance (or part of a group of chemical substances) concerning which it has been proposed that use within the European Union be subject to authorisation under the REACH Regulation. Indeed, listing of a substance as an SVHC by the European Chemicals Agency (ECHA) is the first step in the procedure for authorisation or restriction of use of a chemical. The first list of SVHCs was published on 28 October 2008 and the list has been updated many times to include new candidates. The most recent update occurred in January 2022 to include a total of 223 SVHC. Criteria The criteria are given in article 57 of the REACH Regulation. A substance ''may'' be proposed as an SVHC if it meets one or more of the following criteria: *it is carcinogenic; *it is mutagenic; *it is toxic for reproduction; *it is persistent, bioaccumulative and toxicAnnex XIII, REACH Regulation, at pp. 383–85. (PBT substances); *it is very persistent and very bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
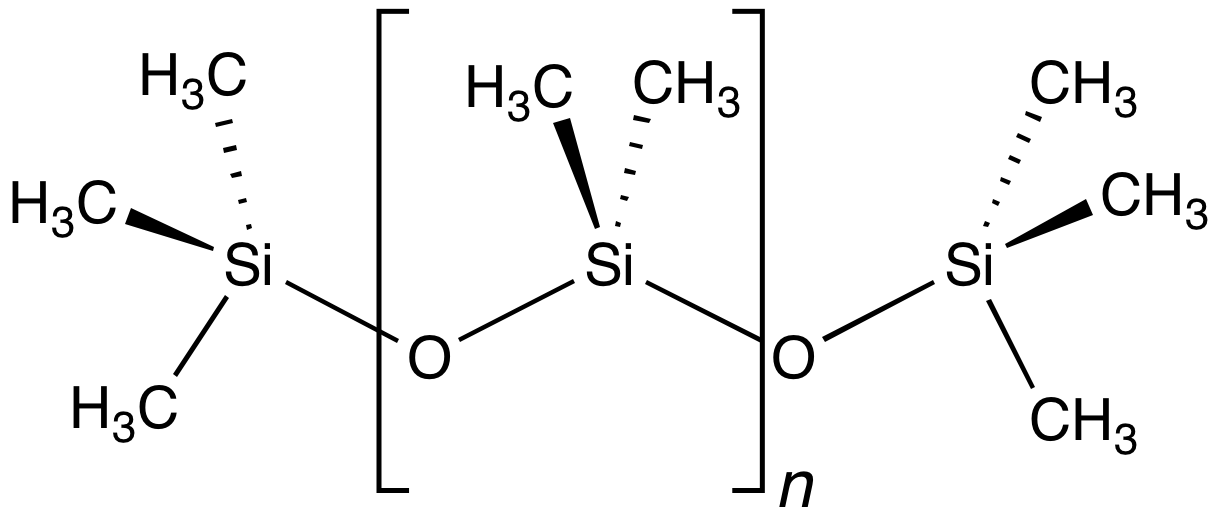
Siloxane
A siloxane is a functional group in organosilicon chemistry with the Si−O−Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)''n''OH and (OSiH2)n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen (O2-) atom. The siloxane functional group forms the backbone of silicones, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− (where the three Rs may be different) is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility. Structure Siloxanes generally adopt structures expected for linked tetrahedral ("''sp''3-like") centers. The Si−O bond length is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si-O-Si angle is rather open at 142.5°. By contrast, the C−O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Masterbatch
Masterbatch (MB) is a solid additive used for coloring (color masterbatch) or imparting other properties (additive masterbatch) to plastics. Masterbatch is a concentrated mixture of pigments and/or additives which is manufactured by encapsulation during a heat process or twin screw extrusion into a carrier matrixresin, which is then cooled and further cut into a granular shape. Masterbatch allows the processor to color raw polymer economically. The alternatives to using masterbatches are buying a fully compounded material (which may be more expensive and less open to e.g. color variability of the product), or compounding from raw materials on site (which is prone to issues with achieving full dispersion of the colorants and additives, and prone to preparing more material than what is used for the production run). In comparison with pure pigments, masterbatches require more storage space and their lead times are longer. Another disadvantage is additional exposure of heat ("heat histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C-N=C angle is 134.9° and the N=C=O angle is 173.1°. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic Compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds ( alkynes). If other elements (heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. The least complex aliphatic compound is methane (CH4). Properties Most aliphatic compounds are flammable, allowing the use of hydrocarbons as fuel, such as methane in Bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoplastic Elastomers
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers, are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric properties. While most elastomers are thermosets, thermoplastics are in contrast relatively easy to use in manufacturing, for example, by injection moulding. Thermoplastic elastomers show advantages typical of both rubbery materials and plastic materials. The benefit of using thermoplastic elastomers is the ability to stretch to moderate elongations and return to its near original shape creating a longer life and better physical range than other materials. The principal difference between thermoset elastomers and thermoplastic elastomers is the type of cross-linking bond in their structures. In fact, crosslinking is a critical structural factor which imparts high elastic properties. Types There are six generic classes of commercial TPEs (design ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |