|
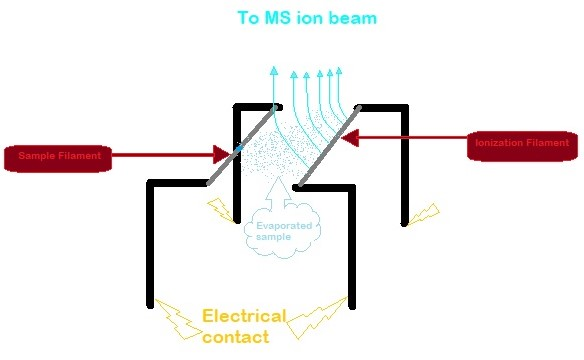
Thermal Ionization Mass Spectrometry
Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive isotope mass spectrometry characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemental analysis of a sample. Singly charged ions of the sample are formed by the thermal ionization effect. A chemically purified liquid sample is placed on a metal filament which is then heated to evaporate the solvent. The removal of an electron from the purified sample is consequently achieved by heating the filament enough to release an electron, which then ionizes the atoms of the sample. TIMS utilizes a magnetic sector mass analyzer to separate the ions based on their mass to charge ratio. The ions gain velocity by an electrical potential gradient and are focused into a beam by electrostatic lenses. The ion beam then passes through the magnetic field of the electromagnet where it is partitioned into separate ion beams based on the io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
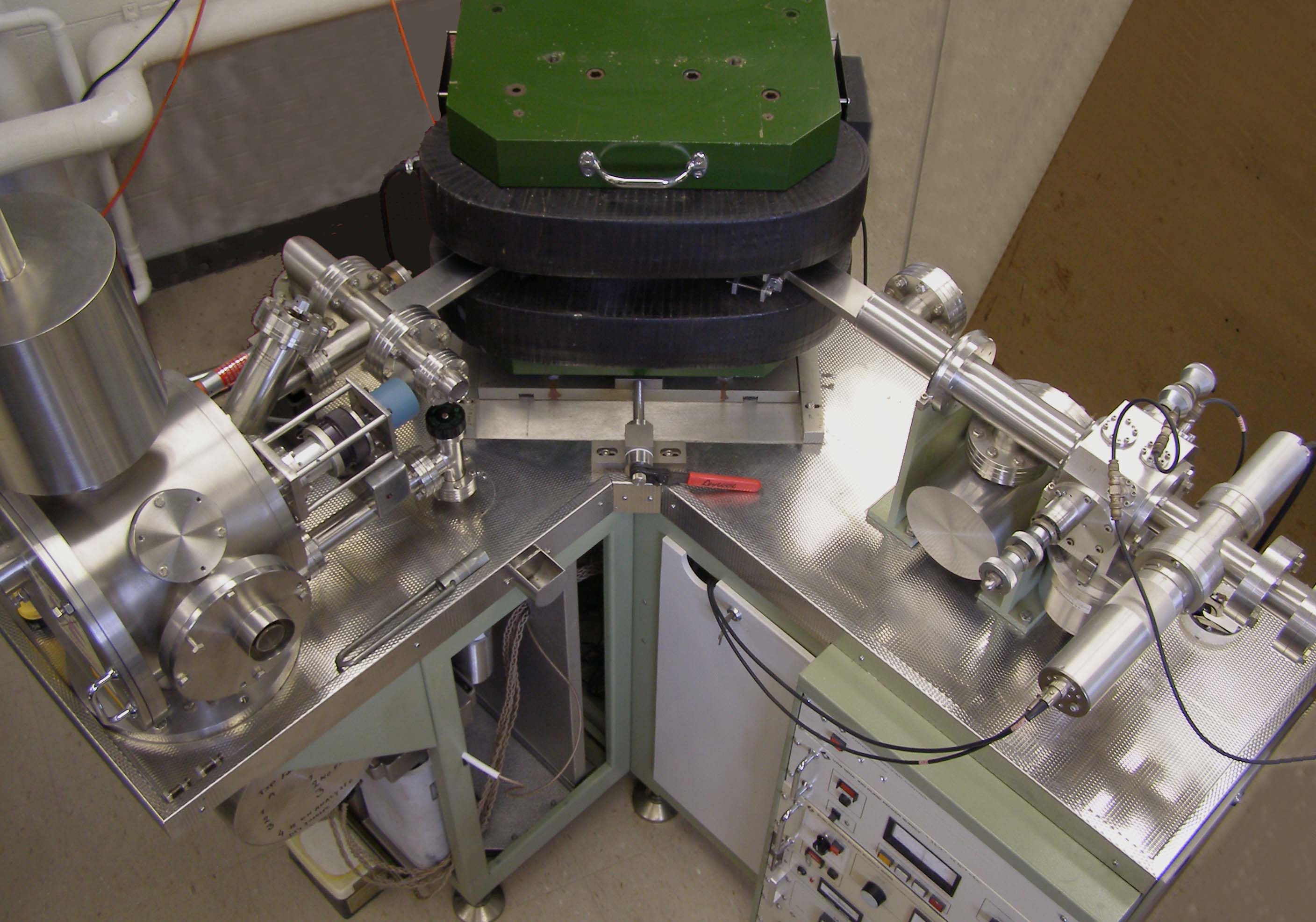
Thermal Ionization Mass Spectrometer
Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive isotope mass spectrometry characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemental analysis of a sample. Singly charged ions of the sample are formed by the thermal ionization effect. A chemically purified liquid sample is placed on a metal filament which is then heated to evaporate the solvent. The removal of an electron from the purified sample is consequently achieved by heating the filament enough to release an electron, which then ionizes the atoms of the sample. TIMS utilizes a Sector instrument, magnetic sector mass analyzer to separate the ions based on their mass to charge ratio. The ions gain velocity by an electrical potential gradient and are focused into a beam by electrostatic lenses. The ion beam then passes through the magnetic field of the electromagnet where it is partitioned into separate ion bea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daly Detector
A Daly detector is a gas-phase ion detector that consists of a metal "doorknob", a scintillator (phosphor screen) and a photomultiplier.N. R. DalyScintillation Type Mass Spectrometer ion Detector. ''Rev. Sci. Instrum.'' 31(3), 264–267 (1960). It was named after its inventor Norman Richard Daly. Daly detectors are typically used in mass spectrometers. Principle of operation Ions that hit the doorknob release secondary electrons. A high voltage (about ) between the doorknob and the scintillator accelerates the electrons onto the phosphor screen, where they are converted to photons. These photons are detected by the photomultiplier. The advantage of the Daly detector is that the photomultiplier can be separated by a window, which lets the photons through from the high vacuum of the mass spectrometer, thus preventing an otherwise possible contamination and extending life span of the detector. The Daly detector also allows a higher acceleration after the field-free region of a time- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Dilution
Isotope dilution analysis is a method of determining the quantity of chemical substances. In its most simple conception, the method of isotope dilution comprises the addition of known amounts of isotopically enriched substance to the analyzed sample. Mixing of the isotopic standard with the sample effectively "dilutes" the isotopic enrichment of the standard and this forms the basis for the isotope dilution method. Isotope dilution is classified as a method of internal standardisation, because the standard (isotopically enriched form of analyte) is added directly to the sample. In addition, unlike traditional analytical methods which rely on signal intensity, isotope dilution employs signal ratios. Owing to both of these advantages, the method of isotope dilution is regarded among chemistry measurement methods of the highest metrological standing. Isotopes are variants of a particular chemical element which differ in neutron number. All isotopes of a given element have the same num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geochronology
Geochronology is the science of determining the age of rocks, fossils, and sediments using signatures inherent in the rocks themselves. Absolute geochronology can be accomplished through radioactive isotopes, whereas relative geochronology is provided by tools such as paleomagnetism and stable isotope ratios. By combining multiple geochronological (and biostratigraphic) indicators the precision of the recovered age can be improved. Geochronology is different in application from biostratigraphy, which is the science of assigning sedimentary rocks to a known geological period via describing, cataloging and comparing fossil floral and faunal assemblages. Biostratigraphy does not ''directly'' provide an absolute age determination of a rock, but merely places it within an ''interval'' of time at which that fossil assemblage is known to have coexisted. Both disciplines work together hand in hand, however, to the point where they share the same system of naming strata (rock layers) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. ::X(g) + e− → X−(g) + energy Note that this is not the same as the enthalpy change of electron capture ionization, which is defined as negative when energy is released. In other words, the enthalpy change and the electron affinity differ by a negative sign. In solid state physics, the electron affinity for a surface is defined somewhat differently ( see below). Measurement and use of electron affinity This property is used to measure atoms and molecules in the gaseous state only, since in a solid or liquid state their energy levels would be changed by contact with other atoms or molecules. A list of the electron affinities was used by Robert S. Mulliken to develop an electronegativity scale for atoms, equal to the average of the electrons affinity and ionization potential. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization Energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ionizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elemental Analysis
Elemental analysis is a process where a sample of some material (e.g., soil, waste or drinking water, bodily fluids, minerals, chemical compounds) is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative (determining what elements are present), and it can be quantitative (determining how much of each is present). Elemental analysis falls within the ambit of analytical chemistry, the instruments involved in deciphering the chemical nature of our world. History Antoine Lavoisier is regarded as the inventor of elemental analysis as a quantitative, experimental tool to assess the chemical composition of a compound. At the time, elemental analysis was based on the gravimetric determination of specific absorbent materials before and after selective adsorption of the combustion gases. Today fully automated systems based on thermal conductivity or infrared spectroscopy detection of the combustion gases, or other spectroscopic methods are us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saha Ionization Equation
In physics, the Saha ionization equation is an expression that relates the ionization state of a gas in thermal equilibrium to the temperature and pressure. The equation is a result of combining ideas of quantum mechanics and statistical mechanics and is used to explain the spectral classification of stars. The expression was developed by Indian physicist Meghnad Saha in 1920. Description For a gas at a high enough temperature (here measured in energy units, i.e. keV or J) and/or density, the thermal collisions of the atoms will ionize some of the atoms, making an ionized gas. When several or more of the electrons that are normally bound to the atom in orbits around the atomic nucleus are freed, they form an independent electron gas cloud co-existing with the surrounding gas of atomic ions and neutral atoms. In turn, this generates an electric field, where the motion of charges generates currents, making a localised magnetic field, and creates the state of matter called plasma. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
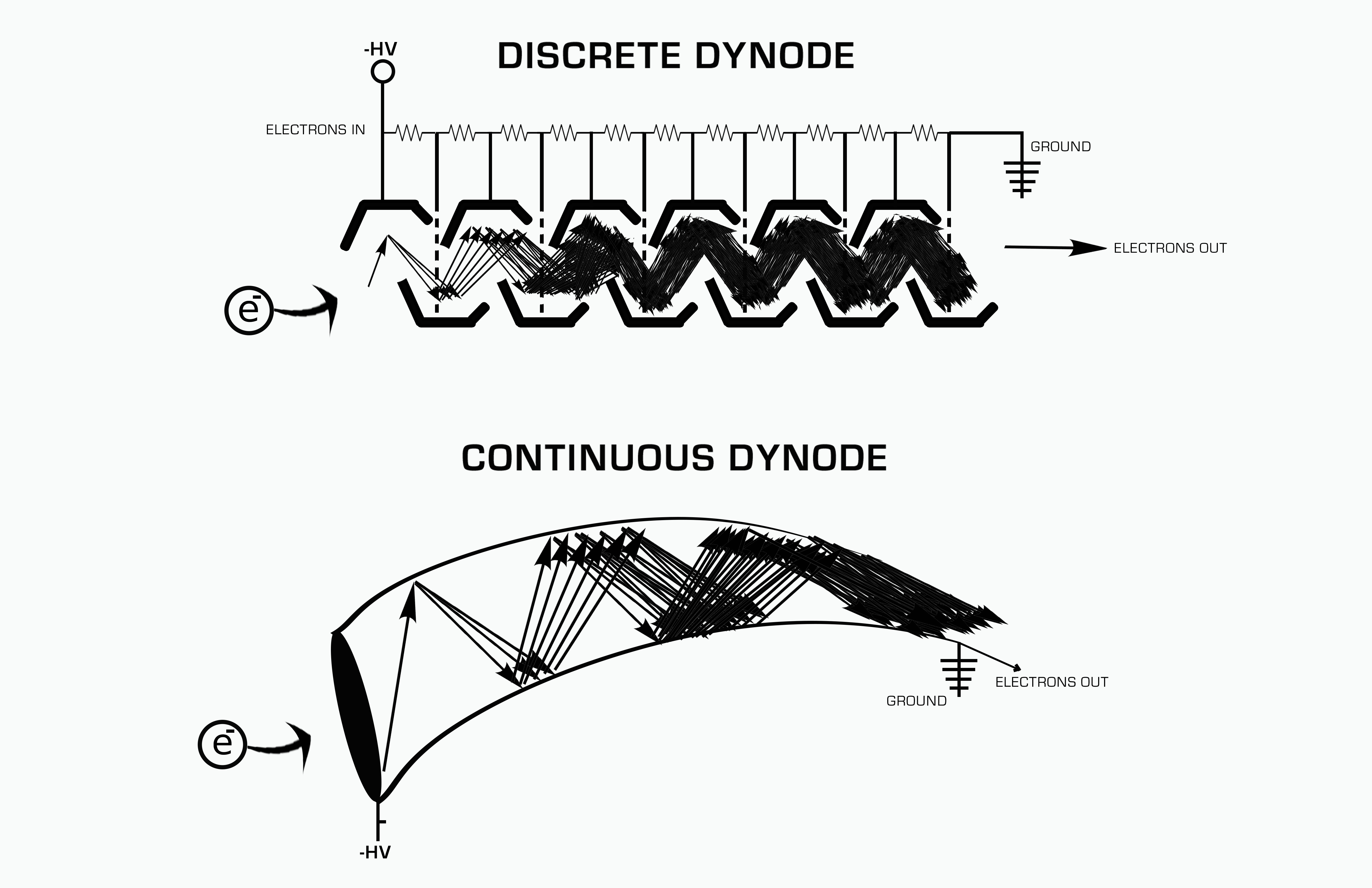
Electron Multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an electric potential is applied between this metal plate and yet another, the emitted electrons will accelerate to the next metal plate and induce secondary emission of still more electrons. This can be repeated a number of times, resulting in a large shower of electrons all collected by a metal anode, all having been triggered by just one. History In 1930, Russian physicist Leonid Aleksandrovitch Kubetsky proposed a device which used photocathodes combined with dynodes, or secondary electron emitters, in a single tube to remove secondary electrons by increasing the electric potential through the device. The electron multiplier can use any number of dynodes in total, which use a coefficient, σ, and created a gain of σn where n is the number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faraday Cup
A Faraday cup is a metal (conductive) cup designed to catch charged particles in vacuum. The resulting current can be measured and used to determine the number of ions or electrons hitting the cup. The Faraday cup was named after Michael Faraday who first theorized ions around 1830. Examples of devices which use Faraday cups include space probes (Voyager 1, & 2, Parker Solar Probe, etc.) and mass spectrometers. Principle of operation When a beam or packet of ions hits the metallic body of the cup, the apparatus gains a small net charge while the ions are neutralized as the charge is transferred to the metal walls. The metal part can then be discharged to measure a small current proportional to the number of impinging ions. The Faraday cup is essentially part of a circuit where ions are the charge carriers in vacuum and it is the interface to the solid metal where electrons act as the charge carriers (as in most circuits). By measuring the electric current (the number of electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |