Thermal Ionization Mass Spectrometer on:
[Wikipedia]
[Google]
[Amazon]
 Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive
Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive
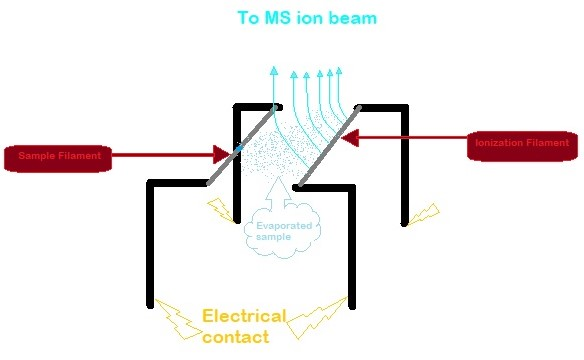 The single filament method is also possible. Once the sample evaporates, the ions can settle back down onto the same filament to get ionized.
The use of a triple filament or multifilament set-up improves ionization efficiency and provides the rate of evaporation and ionization to be controlled separately.
Filaments need to be loaded with activators. An activator represses the evaporation of the desired element and can either increase or decrease the ionization potential of the filament. This results in high ionization efficiency and a higher total yield. The most common activator is silica gel/phosphoric acid for Pb.
The filaments are in a vacuum that can reach temperatures anywhere from 400-2300°C. In order to prevent any damage to the filaments, they are firmly fixed onto a carousel-like sample turret which normally has 10 to 20 filament assemblies. The evaporation process is usually conducted at relatively low temperatures in exchange for long-lasting signals and minor isotopic fractionation. The ionization part requires high temperatures to ensure good ionization efficiency.
The ions emitted have low spatial and energetic spread which makes a single-focusing magnetic sector mass analyzer or quadrupoles suitable. The most common detectors used for TIMS is
The single filament method is also possible. Once the sample evaporates, the ions can settle back down onto the same filament to get ionized.
The use of a triple filament or multifilament set-up improves ionization efficiency and provides the rate of evaporation and ionization to be controlled separately.
Filaments need to be loaded with activators. An activator represses the evaporation of the desired element and can either increase or decrease the ionization potential of the filament. This results in high ionization efficiency and a higher total yield. The most common activator is silica gel/phosphoric acid for Pb.
The filaments are in a vacuum that can reach temperatures anywhere from 400-2300°C. In order to prevent any damage to the filaments, they are firmly fixed onto a carousel-like sample turret which normally has 10 to 20 filament assemblies. The evaporation process is usually conducted at relatively low temperatures in exchange for long-lasting signals and minor isotopic fractionation. The ionization part requires high temperatures to ensure good ionization efficiency.
The ions emitted have low spatial and energetic spread which makes a single-focusing magnetic sector mass analyzer or quadrupoles suitable. The most common detectors used for TIMS is
 Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive
Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemental analysis of a sample. Singly charged ions of the sample are formed by the thermal ionization
Thermal ionization, also known as surface ionization or contact ionization, is a physical process whereby the atoms are desorbed from a hot surface, and in the process are ionized.
Thermal ionization is used to make simple ion sources, for mass s ...
effect. A chemically purified liquid sample is placed on a metal filament which is then heated to evaporate the solvent. The removal of an electron from the purified sample is consequently achieved by heating the filament enough to release an electron, which then ionizes the atoms of the sample. TIMS utilizes a magnetic sector mass analyzer to separate the ions based on their mass to charge ratio. The ions gain velocity by an electrical potential gradient and are focused into a beam by electrostatic lenses. The ion beam then passes through the magnetic field of the electromagnet where it is partitioned into separate ion beams based on the ion's mass/charge ratio. These mass-resolved beams are directed into a detector where it is converted into voltage. The voltage detected is then used to calculate the isotopic ratio.
Ionization source
The filaments used are made fromtantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is ...
(Ta), tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
(W), platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
(Pt) or rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
(Re). Conventionally, there are two filaments used in TIMS. One filament is for the sample and is called the sample filament. The liquid sample is placed on the sample filament which is then evaporated to create ions. Subsequently, these ions land on the other filament, also known as the ionization filament. Here, the ion loses an electron by ionization.
 The single filament method is also possible. Once the sample evaporates, the ions can settle back down onto the same filament to get ionized.
The use of a triple filament or multifilament set-up improves ionization efficiency and provides the rate of evaporation and ionization to be controlled separately.
Filaments need to be loaded with activators. An activator represses the evaporation of the desired element and can either increase or decrease the ionization potential of the filament. This results in high ionization efficiency and a higher total yield. The most common activator is silica gel/phosphoric acid for Pb.
The filaments are in a vacuum that can reach temperatures anywhere from 400-2300°C. In order to prevent any damage to the filaments, they are firmly fixed onto a carousel-like sample turret which normally has 10 to 20 filament assemblies. The evaporation process is usually conducted at relatively low temperatures in exchange for long-lasting signals and minor isotopic fractionation. The ionization part requires high temperatures to ensure good ionization efficiency.
The ions emitted have low spatial and energetic spread which makes a single-focusing magnetic sector mass analyzer or quadrupoles suitable. The most common detectors used for TIMS is
The single filament method is also possible. Once the sample evaporates, the ions can settle back down onto the same filament to get ionized.
The use of a triple filament or multifilament set-up improves ionization efficiency and provides the rate of evaporation and ionization to be controlled separately.
Filaments need to be loaded with activators. An activator represses the evaporation of the desired element and can either increase or decrease the ionization potential of the filament. This results in high ionization efficiency and a higher total yield. The most common activator is silica gel/phosphoric acid for Pb.
The filaments are in a vacuum that can reach temperatures anywhere from 400-2300°C. In order to prevent any damage to the filaments, they are firmly fixed onto a carousel-like sample turret which normally has 10 to 20 filament assemblies. The evaporation process is usually conducted at relatively low temperatures in exchange for long-lasting signals and minor isotopic fractionation. The ionization part requires high temperatures to ensure good ionization efficiency.
The ions emitted have low spatial and energetic spread which makes a single-focusing magnetic sector mass analyzer or quadrupoles suitable. The most common detectors used for TIMS is Faraday cup
A Faraday cup is a metal (conductive) cup designed to catch charged particles in vacuum. The resulting current can be measured and used to determine the number of ions or electrons hitting the cup. The Faraday cup was named after Michael Faraday w ...
, Daly detector
A Daly detector is a gas-phase ion detector that consists of a metal "doorknob", a scintillator (phosphor screen) and a photomultiplier.N. R. DalyScintillation Type Mass Spectrometer ion Detector. ''Rev. Sci. Instrum.'' 31(3), 264–267 (1960). I ...
, and electron multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an ele ...
. Customarily, TI ion sources are assembled with multicollector (MC) systems.
Thermal ionization mechanism
When the hot filament heats the liquid sample, the fermi levels within the sample reaches parity with that of the metal. In turn, this allows for an electron to tunnel from the sample to the metal filament. As a result, positive ions are formed from the sample that lost an electron. This transferring of electrons also result in the formation of negative ions. Subsequently, there are two types of thermal ionizations. One is positive thermal ionization (P-TI) and the second is negative thermal ionization (N-TI). The production of ions is parameterized by theSaha ionization equation
In physics, the Saha ionization equation is an expression that relates the ionization state of a gas in thermal equilibrium to the temperature and pressure. The equation is a result of combining ideas of quantum mechanics and statistical mechanics ...
or the Saha-Langmuir equation.
Isotope ratio measurement
The relative abundances of different isotopes are then used to describe the chemical fractionation of different isotopes, travel in different reservoirs of non-radiogenic isotopes, and age or origins of solar system objects by the presence of radiogenic daughter isotopes.Lehto, J., X. Hou, 2011. Chemistry and Analysis of Radionuclides. Wiley-VCH.Dickin, A.P., 2005. Radiogenic Isotope Geology 2nd ed. Cambridge: Cambridge University Press. pp. 21-22Elemental analysis
Elemental analysis is a process where a sample of some material (e.g., soil, waste or drinking water, bodily fluids, minerals, chemical compounds) is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualita ...
is a predominant application of TIMS as it gives reliable isotopic ratios. Following the trend of decreasing ionization energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
, elements located towards the bottom left of the periodic table are viable for TIMS. In addition, the high electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
seen towards the upper right of the periodic table makes these nonmetals excellent candidates. The technique is used extensively in isotope geochemistry, geochronology
Geochronology is the science of determining the age of rocks, fossils, and sediments using signatures inherent in the rocks themselves. Absolute geochronology can be accomplished through radioactive isotopes, whereas relative geochronology is pr ...
, and in cosmochemistry.
Quantitative isotope ratio techniques include isotope dilution
Isotope dilution analysis is a method of determining the quantity of chemical substances. In its most simple conception, the method of isotope dilution comprises the addition of known amounts of isotopically enriched substance to the analyzed samp ...
thermal ionization mass spectrometry (ID-TIMS) and chemical abrasion thermal ionization mass spectrometry (CA-TIMS).
Isotope dilution method is used because the signal intensity in TIMS isn't proportional to the amount that is placed into TIMS.
For age dating, mass spectrometers with magnetic sectors have better precision than a quadrupole mass spectrometer or quadrupole mass analyzer
The quadrupole mass analyzer, originally conceived by Nobel Laureate Wolfgang Paul and his student Helmut Steinwedel, also known as quadrupole mass filter, is one type of mass analyzer used in mass spectrometry. As the name implies, it consists of ...
. Inductively coupled plasma-quadrupole mass spectrometers allows for an even higher precision of detecting the change of isotopic ratios by radioactive decay. The more precision means the higher resolution in age dating.
References
{{mass spectrometry Mass spectrometry