|
Thermal Grease
Thermal paste (also called thermal compound, thermal grease, thermal interface material (TIM), thermal gel, heat paste, heat sink compound, heat sink paste or CPU grease) is a thermally conductive (but usually electrically insulating) chemical compound, which is commonly used as an interface between heat sinks and heat sources such as high-power semiconductor devices. The main role of thermal paste is to eliminate air gaps or spaces (which act as thermal insulation) from the interface area in order to maximize heat transfer and dissipation. Thermal paste is an example of a thermal interface material. As opposed to thermal adhesive, thermal paste does not add mechanical strength to the bond between heat source and heat sink. It has to be coupled with a mechanical fixation mechanism such as screws to hold the heat sink in place and to apply pressure, spreading and thinning the thermal paste. Composition Thermal paste consists of a polymerizable liquid matrix and large vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Greases
A thermal column (or thermal) is a rising mass of buoyant air, a convective current in the atmosphere, that transfers heat energy vertically. Thermals are created by the uneven heating of Earth's surface from solar radiation, and are an example of convection, specifically atmospheric convection. Thermals on Earth The Sun warms the ground, which in turn warms the air directly above. The warm air near the surface expands, becoming less density, dense than the surrounding air. The lighter air rises and cools due to its expansion in the lower pressure at higher altitudes. It stops rising when it has cooled to the same temperature, thus density, as the surrounding air. Associated with a thermal is a downward flow surrounding the thermal column. The downward-moving exterior is caused by colder air being displaced at the top of the thermal. The size and Power (physics), strength of thermals are influenced by the properties of the lower atmosphere (the ''troposphere''). When the air ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk. Chemistry More precisely called polymerized siloxanes or polysiloxanes, silicones consist of an inorganic silicon–oxygen backbone chain (⋯−Si−O−Si−O−Si−O−⋯) with two organic groups attached to each silicon center. Commonly, the organic groups are methyl. The materials can be cyclic or polymeric. By varying the −Si−O− chain lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lower melting point of , well below the freezing point of water, is claimed for the alloy galinstan (62–� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galinstan
Galinstan (R) is a brand name for a alloy composed of gallium, indium, and tin which melts at and is thus liquid at room temperature. However, it is not a eutectic alloy but a near eutectic alloy. In scientific literature, galinstan is also used as an acronym denoting the eutectic composition of the alloy of Ga-In-Sn, which melts at around . The composition of both alloys is roughly the same, albeit the Galinstan (R), the technical product of a company, has likely additions of flux to improve flowability, reduce melting temperature, and reduce surface tension. The physical properties of the Galinstan (R) and the pure eutectic alloy EGaInSn thus differ slightly. Galinstan is composed of 68.5%Ga, 21.5%In, and 10.0%Sn (by weight). Due to the low toxicity and low reactivity of its component metals, in many applications, galinstan has replaced the toxic liquid mercury or the reactive NaK (sodium–potassium alloy). Name The name "Galinstan" is a portmanteau of gallium, indium, and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Metal
A liquid metal is a metal or a metal alloy which is liquid at or near room temperature. The only stable liquid elemental metal at room temperature is Mercury (element), mercury (Hg), which is molten above −38.8 °C (234.3 K, −37.9 °F). Three more stable elemental metals melt just above room temperature: caesium (Cs), which has a melting point of 28.5 °C (83.3 °F); gallium (Ga) (30 °C [86 °F]); and rubidium (Rb) (39 °C [102 °F]). The radioactive metal francium (Fr) is probably liquid close to room temperature as well. Calculations predict that the radioactive metals copernicium (Cn) and flerovium (Fl) should also be liquid at room temperature. Alloys can be liquid if they form a eutectic, meaning that the alloy's melting point is lower than any of the alloy's constituent metals. The standard metal for creating liquid alloys used to be mercury (element), mercury, but gallium-based alloys, which are lower both in their vapor press ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micronized
Micronization is the process of reducing the average diameter of a solid material's particles. Traditional techniques for micronization focus on mechanical means, such as milling and grinding. Modern techniques make use of the properties of supercritical fluids and manipulate the principles of solubility. The term micronization usually refers to the reduction of average particle diameters to the micrometer range, but can also describe further reduction to the nanometer scale. Common applications include the production of active chemical ingredients, foodstuff ingredients, and pharmaceuticals. These chemicals need to be micronized to increase efficacy. Traditional techniques Traditional micronization techniques are based on friction to reduce particle size. Such methods include milling, bashing and grinding. A typical industrial mill is composed of a cylindrical metallic drum that usually contains steel spheres. As the drum rotates the spheres inside collide with the particles of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann's Encyclopedia Of Industrial Chemistry
''Ullmann's Encyclopedia of Industrial Chemistry'' is a major reference work related to industrial chemistry by Chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie" until 1984. History Ullmann's Encyclopedia of Industrial Chemistry is a major reference work related to industrial chemistry by chemist Fritz Ullmann. Its 1st edition was published in German by Fritz Ullmann in 1914. The 4th edition, published 1972 to 1984, already contained 25 volumes. The 5th edition, published 1985 to 1996, was the first version available in English. In 1997, the first online version was published. 2014 marked its centenary. As of 2016, Ullmann's Encyclopedia was in its 7th edition, in 40 volumes including one index volume and more than 1,050 articles (200 more than the 6th edition), approx. 30,000 pages, 22,000 images, 8,000 tables, 19,000 references and 85,000 indices. Editions * 1914–1922: 1st edition in 12 volumes, which can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum Nitride
Aluminium nitride ( Al N) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies. History and physical properties AlN was first synthesized in 1862 by F. Briegleb and A. Geuther. AlN, in the pure (undoped) state has an electrical conductivity of 10−11–10−13 Ω−1⋅cm−1, rising to 10−5–10−6 Ω−1⋅cm−1 when doped. Electrical breakdown occurs at a field of 1.2–1.8 V/mm (dielectric strength). The material exists primarily in the hexagonal wurtzite crystal structure, but also has a metastable cubic zincblende phase, which is synthesized primarily in the form of thin films. It is predicted that the cubic phase of AlN (zb-AlN) can exhibit superconductivity at high pressures. In AlN wurtzite crystal structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. ZnO is a wide-band gap semiconductor of the II-VI semiconductor group. The native doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility, wide band gap, and strong room-temperature luminescence. Those properties make ZnO valuable for a variety of emerging applications: transparent electrodes in liquid crystal displays, energy-saving or heat-protecting windows, and electronics as thin-film transistors and lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
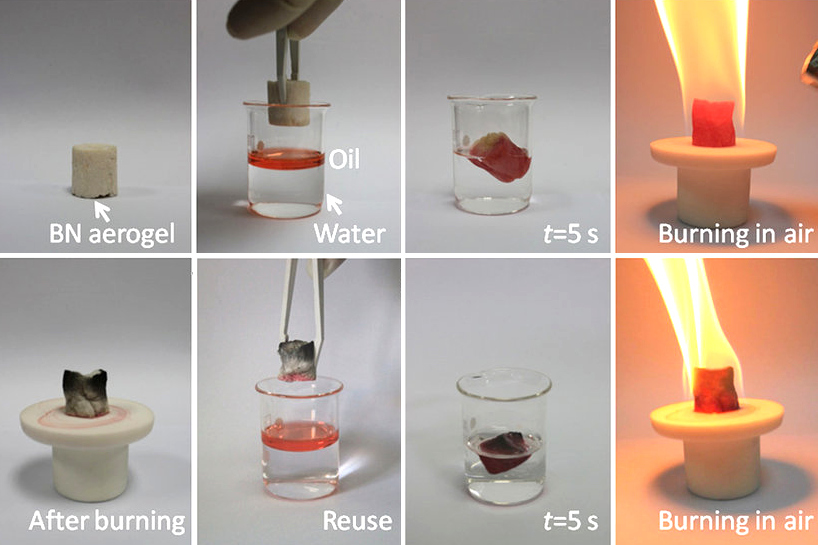
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varyin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |