|
Thallium(I) Oxide
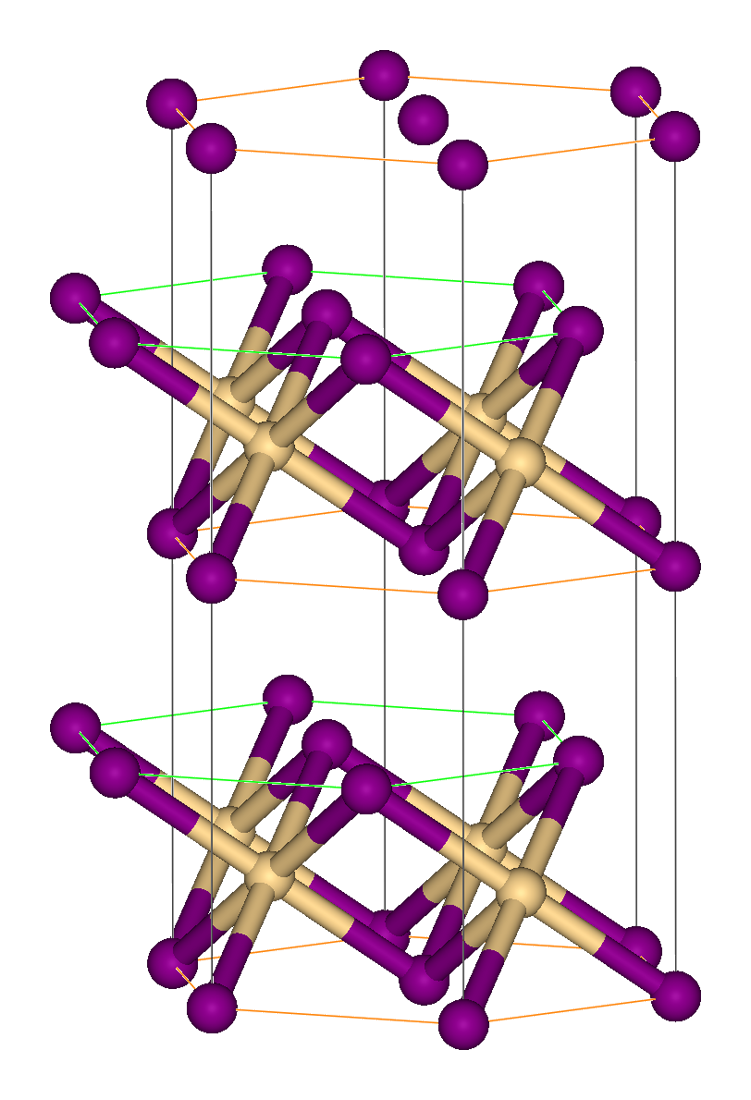
Thallium(I) oxide is the inorganic compound of thallium and oxygen with the formula Tl2O in which thallium is in its +1 oxidation state. It is black and produces a base (chemistry), basic yellow solution of thallium(I) hydroxide (TlOH) when dissolved in water. It is formed by heating solid TlOH or Tl2CO3 in the absence of air. Thallium oxide is used to make special high refractive index glass. Thallium oxide is a component of several High-temperature superconductivity, high temperature superconductors. Thallium(I) oxide reacts with acids to make thallium(I) salts. Tl2O adopts the anti-cadmium iodide structure in the solid state. In this way, the Tl(I) centers are pyramidal and the oxide centers are octahedral molecular geometry, octahedral. Thallium(I) oxide, like all thallium compounds, is highly toxic. Preparation Thallium(I) oxide can be produced by decomposition of thallium(I) hydroxide at 100 °C or by heating thallium(III) oxide in the absence of air to 700&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thallium(III) Oxide
Thallium(III) oxide, also known as thallic oxide, is a chemical compound of thallium and oxygen. It occurs in nature as the rare mineral avicennite. Its structure is related to that of Mn2O3 which has a bixbyite like structure. Tl2O3 is metallic with high conductivity and is a degenerate n-type semiconductor which may have potential use in solar cells. A method of producing Tl2O3 by MOCVD is known. Any practical use of thallium(III) oxide will always have to take account of thallium's poisonous nature. Contact with moisture and acids may form poisonous thallium compounds. Production It is produced by the reaction of thallium with oxygen or hydrogen peroxide in an alkaline thallium(I) solution. Alternatively, it can be created by the oxidation of thallium(I) nitrate by chlorine in an aqueous potassium hydroxide Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix ''octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-temperature Superconductivity
High-temperature superconductors (abbreviated high-c or HTS) are defined as materials that behave as superconductors at temperatures above , the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previously known superconductors, which function at even colder temperatures close to absolute zero. In absolute terms, these "high temperatures" are still far below ambient, and therefore require cooling. The first high-temperature superconductor was discovered in 1986, by IBM researchers Bednorz and Müller, who were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-c materials are type-II superconductors. The major advantage of high-temperature superconductors is that they can be cooled by using liquid nitrogen, as opposed to the previously known superconductors which require expensive and hard-to-handle coolants, primarily liquid helium. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-Crystallinity, crystalline, often transparency and translucency, transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the Melting, molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silicon dioxide, silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and glasses, eyeglasses, are so commonly made of silicate- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractive Index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium. The refractive index determines how much the path of light is bent, or refracted, when entering a material. This is described by Snell's law of refraction, , where ''θ''1 and ''θ''2 are the angle of incidence and angle of refraction, respectively, of a ray crossing the interface between two media with refractive indices ''n''1 and ''n''2. The refractive indices also determine the amount of light that is reflected when reaching the interface, as well as the critical angle for total internal reflection, their intensity (Fresnel's equations) and Brewster's angle. The refractive index can be seen as the factor by which the speed and the wavelength of the radiation are reduced with respect to their vacuum values: the speed of light in a medium is , and similarly the wavelength in that medium is , where ''� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thallium(I) Hydroxide
Thallium(I) hydroxide, also called thallous hydroxide, TlOH, is a hydroxide of thallium, with thallium in oxidation state +1. Synthesis Thallium(I) hydroxide is obtained from the decomposition of thallium(I) ethoxide Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and C ... in water. :C2H5OTl + H2O → TlOH + C2H5OH This can also be done by direct reaction of thallium with ethanol and oxygen gas. :4 Tl + 2 C2H5OH + O2 → 2 C2H5OTl + 2 TlOH Another method is the reaction between thallium(I) sulfate and barium hydroxide. :Tl2SO4 + Ba(OH)2 → 2 TlOH + BaSO4 Properties Thallous hydroxide is a strong base; it dissociates to the thallous ion, Tl+, except in strongly basic conditions. Tl+ resembles an alkali metal ion, A+, such as Li+ or K+. References Hydroxides Thall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |