|
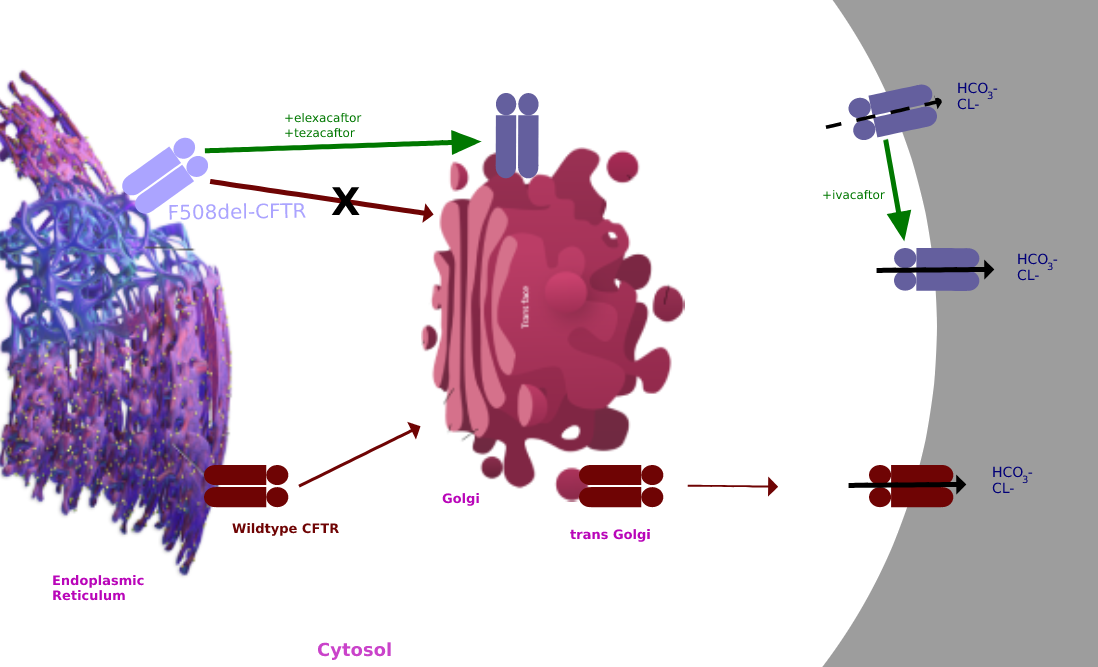
Tezacaftor Ivacaftor
Tezacaftor is a drug used for the treatment of cystic fibrosis (CF) in people six years and older, who have a F508del mutation, the most common type of mutation in the CFTR gene. It is sold as a fixed-dose combination with ivacaftor under the brand name Symdeko. It was approved by the U.S. FDA in 2018. The combination of elexacaftor, tezacaftor, and ivacaftor is being sold as Trikafta. In 2019, the U.S. Food and Drug Administration (FDA) approved a combination of elexacaftor, tezacaftor, and ivacaftor. Mechanism of action Tezacaftor acts as a corrector to help the folding and presentation of the CFTR protein to the cell surface, which improves its function for individuals with a F508del mutation. Clinical trials The EVOLVE and EXPAND study findings were published in 2017. EVOLVE trial The EVOLVE trial analyzed tezacaftor/ivacaftor in patients with cystic fibrosis, specifically with the homozygous for Phe508del mutation. The EVOLVE trial is a phase 3, double-blinded, mult ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trikafta
Elexacaftor/tezacaftor/ivacaftor, sold under the brand names Trikafta (US) and Kaftrio (EU), is a fixed-dose combination medication used to treat cystic fibrosis. Elexacaftor/tezacaftor/ivacaftor is composed of a combination of ivacaftor, a chloride channel opener, and elexacaftor and tezacaftor, CFTR modulators. It is approved for use in the United States for people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. It is also approved for use in Canada, the European Union and Australia. Medical uses The combination is indicated for the treatment of people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. Side effects The most common side effects affecting more than 5% of patients are headache, upper respiratory tract infection, abdominal pain, diarrhea, rash, alanine aminotransferase increase, nasal congestion, blood creatine phosphokinase increase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ivacaftor
Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (primarily the G551D mutation), who account for 4–5% cases of cystic fibrosis. It is also included in combination medications, lumacaftor/ivacaftor, tezacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor which are used to treat people with cystic fibrosis. Ivacaftor was developed by Vertex Pharmaceuticals in conjunction with the Cystic Fibrosis Foundation and is the first medication that treats the underlying cause rather than the symptoms of the disease. It was approved by the U.S. Food and Drug Administration (FDA) in January 2012. It is one of the most expensive drugs, costing over per year, which has led to criticism of the high cost. The combination drug lumacaftor/ivacaftor was approved by the FDA in July 2015. Cystic fibrosis is caused by any one of several defects in the CFTR protein, which regulates fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like Saline (medicine), saline), sham surgery, and other procedures. In general, placebos can affect how patients perceive their condition and encourage the body's chemical processes for relieving pain and a few other symptoms, but have no impact on the disease itself. Improvements that patients experience after being treated with a placebo can also be due to unrelated factors, such as regression to the mean (a statistical effect where an unusually high or low measurement is likely to be followed by a less extreme one). The use of placebos in clinical medicine raises ethical concerns, especially if they are disguised as an active treatment, as this introduces dishonesty into the doctor–patient relationship and bypasses informed consent. While it was once assumed that this deception was necessary for placebos to have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indoles
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanes
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into clin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vertex Pharmaceuticals Incorporated
Vertex Pharmaceuticals is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headquarters in South Boston, Massachusetts, and three research facilities, in San Diego, California, and Milton Park, near Oxford, England. History Vertex was founded in 1989 by Joshua Boger and Kevin J. Kinsella . to ''"transform the way serious diseases are treated."'' The company's beginnings were profiled by Barry Werth in the 1994 book, '' The Billion-Dollar Molecule''. His 2014 book, ''The Antidote: Inside the World of New Pharma'', chronicled the company's subsequent development over the next two decades. By 2004, its product pipeline focused on viral infections, inflammatory and autoimmune disorders, and cancer. In 2009, the company had about 1,800 employees, including 1,200 in the Boston area. By 2019 there were about 2,500 employ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New England Journal Of Medicine
''The New England Journal of Medicine'' (''NEJM'') is a weekly medical journal published by the Massachusetts Medical Society. It is among the most prestigious peer-reviewed medical journals as well as the oldest continuously published one. History In September 1811, John Collins Warren, a Boston physician, along with James Jackson, submitted a formal prospectus to establish the ''New England Journal of Medicine and Surgery and Collateral Branches of Science'' as a medical and philosophical journal. Subsequently, the first issue of the ''New England Journal of Medicine and Surgery and the Collateral Branches of Medical Science'' was published in January 1812. The journal was published quarterly. In 1823, another publication, the ''Boston Medical Intelligencer'', appeared under the editorship of Jerome V. C. Smith. The editors of the ''New England Journal of Medicine and Surgery and the Collateral Branches of Medical Science'' purchased the weekly ''Intelligencer'' for $600 in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase 3 Clinical Testing
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |