|
Tetrasulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anions, which have the general formula . These anions are the conjugate bases of the hydrogen polysulfides . Organic polysulfides generally have the formulae , where R = alkyl or aryl. Polysulfide salts and complexes The alkali metal polysulfides arise by treatment of a solution of sulfide, e.g. sodium sulfide, with elemental sulfur: : In some cases, these anions have been obtained as organic salts, which are soluble in organic solvents. The energy released in the reaction of sodium and elemental sulfur is the basis of battery technology. The sodium–sulfur battery and the lithium–sulfur battery require high temperatures to maintain liquid polysulfide and -conductive membranes that are unreactive toward sodium, sulfur, and sodium sulfide. Polysulfides are ligands in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation Polymerization
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all Degree of polymerization, degrees of polymerization, or by condensative Chain-growth polymerization, chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers. Condensation polymerization is a form of step-growth polymerization. Linear polymers are produced from bifunctional monomers, i.e. compounds with two reactive end-groups. Common condensation polymers include polyamides, polyacetals, and proteins. Polyamides One important class of condensation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neoprene
Neoprene (also polychloroprene) is a family of synthetic rubbers that are produced by polymerization of chloroprene.Werner Obrecht, Jean-Pierre Lambert, Michael Happ, Christiane Oppenheimer-Stix, John Dunn and Ralf Krüger "Rubber, 4. Emulsion Rubbers" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. Neoprene exhibits good chemical stability and maintains flexibility over a wide temperature range. Neoprene is sold either as solid rubber or in latex form and is used in a wide variety of commercial applications, such as laptop sleeves, orthopaedic braces (wrist, knee, etc.), electrical insulation, liquid and sheet-applied elastomeric membranes or flashings, and automotive fan belts. Production Neoprene is produced by free-radical polymerization of chloroprene. In commercial production, this polymer is prepared by free radical emulsion polymerization. Polymerization is initiated using potassium persulfate. Bifunctional nucleophiles, metal oxides (e.g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroprene
Chloroprene is the common name for 2-chlorobuta-1,3-diene (IUPAC name) with the chemical formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber. History Although it may have been discovered earlier, chloroprene was largely developed by DuPont during the early 1930s, specifically with the formation of neoprene in mind. The chemists Elmer K. Bolton, Wallace Carothers, Arnold Collins and Ira Williams are generally accredited with its development and commercialisation although the work was based upon that of Julius Arthur Nieuwland, with whom they collaborated. Production Chloroprene is produced in three steps from 1,3-butadiene: (i) chlorination, (ii) isomerization of part of the product stream, and (iii) dehydrochlorination of 3,4-dichlorobut-1-ene. Chlorine adds to 1,3-butadiene to afford a mixture of 3,4-dichlorobut-1-ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
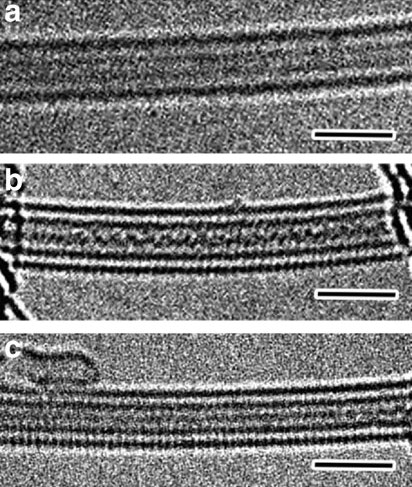
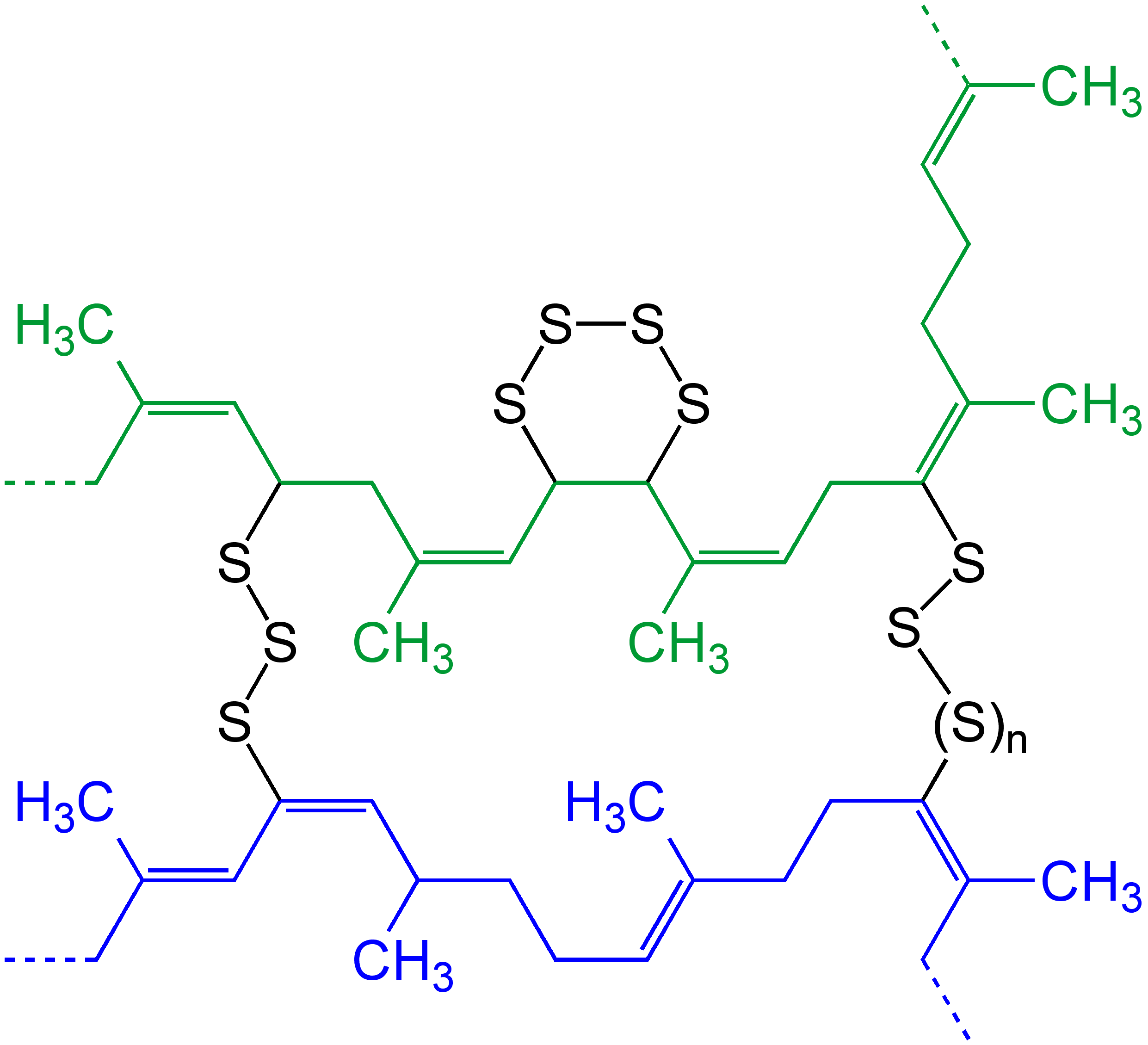
Vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides. Vulcanization can be defined as the curing of elastomers, with the terms 'vulcanization' and 'curing' sometimes used interchangeably in this context. It works by forming cross-links between sections of polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electrical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible. The word vulcanization is derived from Vulcan, the Roman god of fire and forge. History Rubber—latex� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and Indonesia are three of the leading rubber producers. Types of polyisoprene that are used as natural rubbers are classified as elastomers. Currently, rubber is harvested mainly in the form of the latex from the rubber tree (''Hevea brasiliensis'') or others. The latex is a sticky, milky and white colloid drawn off by making incisions in the bark and collecting the fluid in vessels in a process called "tapping". The latex then is refined into the rubber that is ready for commercial processing. In major areas, latex is allowed to coagulate in the collection cup. The coagulated lumps are collected and processed into dry forms for sale. Natural rubber is used extensively in many applications and products, either alone or in combination wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curing (chemistry)
Curing is a chemical process employed in polymer chemistry and process engineering that produces the toughening or hardening of a polymer material by cross-linking of polymer chains. Even if it is strongly associated with the production of thermosetting polymers, the term "curing" can be used for all the processes where a solid product is obtained from a liquid solution, such as with PVC plastisols. Curing process During the curing process, single monomers and oligomers, mixed with or without a curing agent, react to form a tridimensional polymeric network. In the very first part of the reaction branches of molecules with various architectures are formed, and their molecular weight increases in time with the extent of the reaction until the network size is equal to the size of the system. The system has lost its solubility and its viscosity tends to infinite. The remaining molecules start to coexist with the macroscopic network until they react with the network creating other c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic polymer'', is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant ( E ≈ 3 M Pa) and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vulcanization Of POLYIsoprene V
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides. Vulcanization can be defined as the curing of elastomers, with the terms 'vulcanization' and 'curing' sometimes used interchangeably in this context. It works by forming cross-links between sections of polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electrical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible. The word vulcanization is derived from Vulcan, the Roman god of fire and forge. History Rubber—latex— ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly(p-phenylene Sulfide)
Polyphenylene sulfide (PPS) is an organic polymer consisting of aromatic rings linked by sulfides. Synthetic fiber and textiles derived from this polymer resist chemical and thermal attack. PPS is used in filter fabric for coal boilers, papermaking felts, electrical insulation, film capacitors, specialty membranes, gaskets, and packings. PPS is the precursor to a conductive polymer of the semi-flexible rod polymer family. The PPS, which is otherwise insulating, can be converted to the semiconducting form by oxidation or use of dopants.David Parker, Jan Bussink, Hendrik T. van de Grampel, Gary W. Wheatley, Ernst-Ulrich Dorf, Edgar Ostlinning, Klaus Reinking, "Polymers, High-Temperature" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH: Weinheim. Polyphenylene sulfide is an engineering plastic, commonly used today as a high-performance thermoplastic. PPS can be molded, extruded, or machined to tight tolerances. In its pure solid form, it may be opaque white to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |