|
Tetrahydrofolate
Tetrahydrofolic acid (THFA), or tetrahydrofolate, is a folic acid derivative. Metabolism Human synthesis Tetrahydrofolic acid is produced from dihydrofolic acid by dihydrofolate reductase. This reaction is inhibited by methotrexate. It is converted into 5,10-methylenetetrahydrofolate by serine hydroxymethyltransferase. Bacterial synthesis Many bacteria use dihydropteroate synthetase to produce dihydropteroate, a molecule without function in humans. This makes it a useful target for sulfonamide antibiotics, which compete with the PABA precursor. Functions Tetrahydrofolic acid is a cofactor in many reactions, especially in the synthesis (or anabolism) of amino acids and nucleic acids. In addition, it serves as a carrier molecule for single-carbon moieties, that is, groups containing one carbon atom e.g. methyl, methylene, methenyl, formyl, or formimino. When combined with one such single-carbon moiety as in 10-formyltetrahydrofolate, it acts as a donor of a group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folic Acid
Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and storage. Folate is required for the body to make DNA and RNA and metabolise amino acids necessary for cell division. As humans cannot make folate, it is required in the diet, making it an essential nutrient. It occurs naturally in many foods. The recommended adult daily intake of folate in the U.S. is 400 micrograms from foods or dietary supplements. Folate in the form of folic acid is used to treat anemia caused by folate deficiency. Folic acid is also used as a supplement by women during pregnancy to reduce the risk of neural tube defects (NTDs) in the baby. Low levels in early pregnancy are believed to be the cause of more than half of babies born with NTDs. More than 80 countries use either mandatory or voluntary fortification of c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5,10-methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer R5,10-methylene-THF. As an intermediate in one-carbon metabolism, 5,10-CH2-THF interconverts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR) It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase. Selected functions Formaldehyde detoxification Methylenetetrahydrofolate is an intermediate in the detoxification of formaldehyde. Pyrimidine biosynthesis It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate ( dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5,10-Methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer R5,10-methylene-THF. As an intermediate in one-carbon metabolism, 5,10-CH2-THF interconverts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR) It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase. Selected functions Formaldehyde detoxification Methylenetetrahydrofolate is an intermediate in the detoxification of formaldehyde. Pyrimidine biosynthesis It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate ( dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Hydroxymethyltransferase
Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme () which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-Methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell. Structure The structure of the SHMT monomer is similar across prokaryotes and eukaryotes, but whereas the active enzyme is a dimer in prokaryotes, the enzyme exists as a tetramer in eukaryotic cells, though the evolutionary basis for this difference in structure is unknown. However, the evolutionary path taken by SHMT going from prokaryotic dimeric form to the eukaryotic tetrameric form can be easily seen as a sort of doubling event. In other words, the eukaryotic SHMT tetramer resembles two prokaryotic dimers that have packed together, forming what has been described as a †... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrofolate Reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the ''DHFR'' gene. It is found in the q11→q22 region of chromosome 5. Bacterial species possess distinct DHFR enzymes (based on their pattern of binding diaminoheterocyclic molecules), but mammalian DHFRs are highly similar. Structure A central eight-stranded beta-pleated sheet makes up the main feature of the polypeptide backbone folding of DHFR. Seven of these strands are parallel and the eighth runs antiparallel. Four alpha helices connect successive beta strands. Residues 9 – 24 are termed "Met20" or "loop 1" and, along with other loops, are part of the major subdomain that surround the active site. The active site is situated in the N-terminal half of the sequence, which includes a conse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrofolic Acid
Dihydrofolic acid (conjugate base dihydrofolate) (DHF) is a folic acid ( vitamin B9) derivative which is converted to tetrahydrofolic acid by dihydrofolate reductase. Since tetrahydrofolate is needed to make both purines and pyrimidines, which are building blocks of DNA and RNA, dihydrofolate reductase is targeted by various drugs to prevent nucleic acid Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ... synthesis. Interactive pathway map Further reading * References Folates {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrofolic Acid
Dihydrofolic acid (conjugate base dihydrofolate) (DHF) is a folic acid ( vitamin B9) derivative which is converted to tetrahydrofolic acid by dihydrofolate reductase. Since tetrahydrofolate is needed to make both purines and pyrimidines, which are building blocks of DNA and RNA, dihydrofolate reductase is targeted by various drugs to prevent nucleic acid Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ... synthesis. Interactive pathway map Further reading * References Folates {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methotrexate
Methotrexate (MTX), formerly known as amethopterin, is a chemotherapy agent and immune-system suppressant. It is used to treat cancer, autoimmune diseases, and ectopic pregnancies. Types of cancers it is used for include breast cancer, leukemia, lung cancer, lymphoma, gestational trophoblastic disease, and osteosarcoma. Types of autoimmune diseases it is used for include psoriasis, rheumatoid arthritis, and Crohn's disease. It can be given by mouth or by injection. Common side effects include nausea, feeling tired, fever, increased risk of infection, low white blood cell counts, and breakdown of the skin inside the mouth. Other side effects may include liver disease, lung disease, lymphoma, and severe skin rashes. People on long-term treatment should be regularly checked for side effects. It is not safe during breastfeeding. In those with kidney problems, lower doses may be needed. It acts by blocking the body's use of folic acid. Methotrexate was first made in 1947 and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydropteroate Synthetase
Dihydropteroate synthase is an enzyme classified under . It produces dihydropteroate in bacteria, but it is not expressed in most eukaryotes including humans. This makes it a useful target for sulfonamide antibiotics, which compete with the PABA precursor. * (2-amino-4-hydroxy-7,8-dihydropteridin-6-yl)methyl diphosphate + 4-aminobenzoate (PABA) \rightleftharpoons diphosphate + dihydropteroate. All organisms require reduced folate cofactors for the synthesis of a variety of metabolites. Most microorganisms must synthesize folate de novo because they lack the active transport system of higher vertebrate cells that allows these organisms to use dietary folates. Proteins containing this domain include dihydropteroate synthase () as well as a group of methyltransferase enzymes including methyltetrahydrofolate, corrinoid iron-sulphur protein methyltransferase (MeTr) that catalyses a key step in the Wood-Ljungdahl pathway of carbon dioxide fixation. Dihydropteroate synthase () (D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
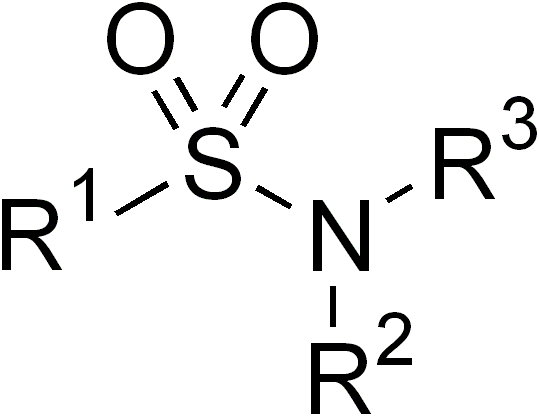
Sulfonamide (medicine)
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides. Allergies to sulfonamides are common. The overall incidence of adverse drug reactions to sulfa antibiotics is approximately 3%, close to penicillin; hence medications containing sulfonamides are prescribed carefully. Sulfonamide drugs were the first broadly effective antibacterials to be used systemically, and paved the way for the antibiotic revolution in medicine. Function In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthase (DHP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |