|
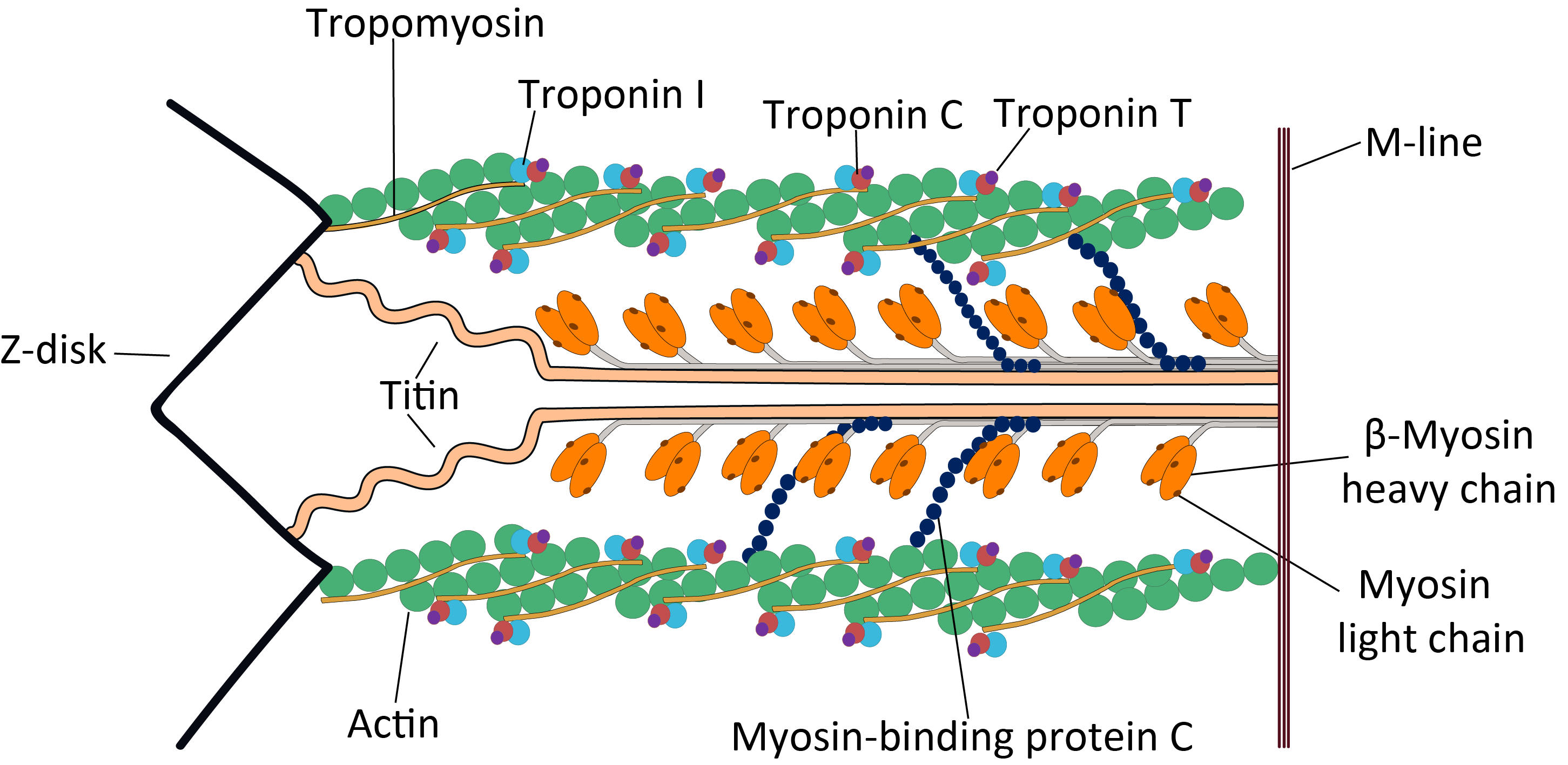
Terminal Web
The terminal web is a filamentous structure found at the apical surface of epithelial cells that possess microvilli. It is composed primarily of actin filaments stabilized by spectrin, which also anchors the terminal web to the apical cell membrane. The presence of myosin II and tropomyosin Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles ar ... helps to explain the contractile ability of the terminal web. When contracted, the terminal web causes a decrease in diameter of the apex of the cell, causing the microvilli, which are anchored into the terminal web through their stiff actin fibers, to spread apart. This spreading apart of the microvilli aids cells in absorption.Ross, Michael H., and Wojciech Pawlina. "Chapter 5: Epithelial Tissue." Histology: a Text and Atlas : with Correlated Ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microvilli
Microvilli (singular: microvillus) are microscopic cellular membrane protrusions that increase the surface area for diffusion and minimize any increase in volume, and are involved in a wide variety of functions, including absorption, secretion, cellular adhesion, and mechanotransduction. Structure Microvilli are covered in plasma membrane, which encloses cytoplasm and microfilaments. Though these are cellular extensions, there are little or no cellular organelles present in the microvilli. Each microvillus has a dense bundle of cross-linked actin filaments, which serves as its structural core. 20 to 30 tightly bundled actin filaments are cross-linked by bundling proteins fimbrin (or plastin-1), villin and espin to form the core of the microvilli. In the enterocyte microvillus, the structural core is attached to the plasma membrane along its length by lateral arms made of myosin 1a and Ca2+ binding protein calmodulin. Myosin 1a functions through a binding site for filamentous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the mobility and contraction of cells during cell division. Actin participates in many important cellular processes, including muscle contraction, cell motility, cell division and cytokinesis, vesicle and organelle movement, cell sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrin
Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin II
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in '' Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles are collectively known as the cytoskeleton, and one of the most ancient systems is based on filamentous polymers of the protein actin. A polymer of a second protein, tropomyosin, is an integral part of most actin filaments in animals. Tropomyosins are a large family of integral components of actin filaments that play a critical role in regulating the function of actin filaments in both muscle and nonmuscle cells. These proteins consist of rod-shaped coiled-coil hetero- or homo- dimers that lie along the α-helical groove of most actin filaments. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. Tropomyosins are often categorised into two groups, muscle tropomyosin isoforms and nonmus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |