|
Tellurite
The tellurite ion is . A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. They are typically colorless or white salts, which in some ways are comparable to sulfite. A mineral with the formula TeO2 is called tellurite. Structure and reactions Tellurite dianion is pyramidal, like selenite and sulfite. The anion has C3v symmetry. Tellurites can be reduced to elemental tellurium by electrolysis In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ... or a strong reducing agent. When fused with nitrate salts, tellurite salts oxidize to tellurates (). Upon acidification of aqueous solutions of tellurite salts, solid hydrated tellurium dioxide (TeO2) precipitates. This reaction allows the separation of tellurium from selenium since selenous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Tellurite
Sodium tellurite is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium. Preparation The main source of tellurium is from copper anode slimes, which contain precious metals as well as various tellurides. These slimes are roasted with sodium carbonate and oxygen to produce sodium tellurite. :Ag2Te + Na2CO3 + O2 → 2Ag + Na2TeO3 + CO2 (400–500 °C) This is a reaction with silver telluride. The telluride is oxidized to tellurite and the silver(I) is reduced to silver. Purification The electrolysis In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ... of a tellurit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Na2TeO3
Sodium tellurite is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium. Preparation The main source of tellurium is from copper anode slimes, which contain precious metals as well as various tellurides. These slimes are roasted with sodium carbonate and oxygen to produce sodium tellurite. :Ag2Te + Na2CO3 + O2 → 2Ag + Na2TeO3 + CO2 (400–500 °C) This is a reaction with silver telluride. The telluride is oxidized to tellurite and the silver(I) is reduced to silver. Purification The electrolysis of a tellurite solution yields purified tellurium. :Anode: 4OH− → 2H2O + O2 + 4e− :Cathode: TeO32− + 3H2O + 4e− → Te + 6OH− Structure and properties Tellurium has properties similar to sulfur and selenium. In the anhydrous form Na2TeO3 the tellurium at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
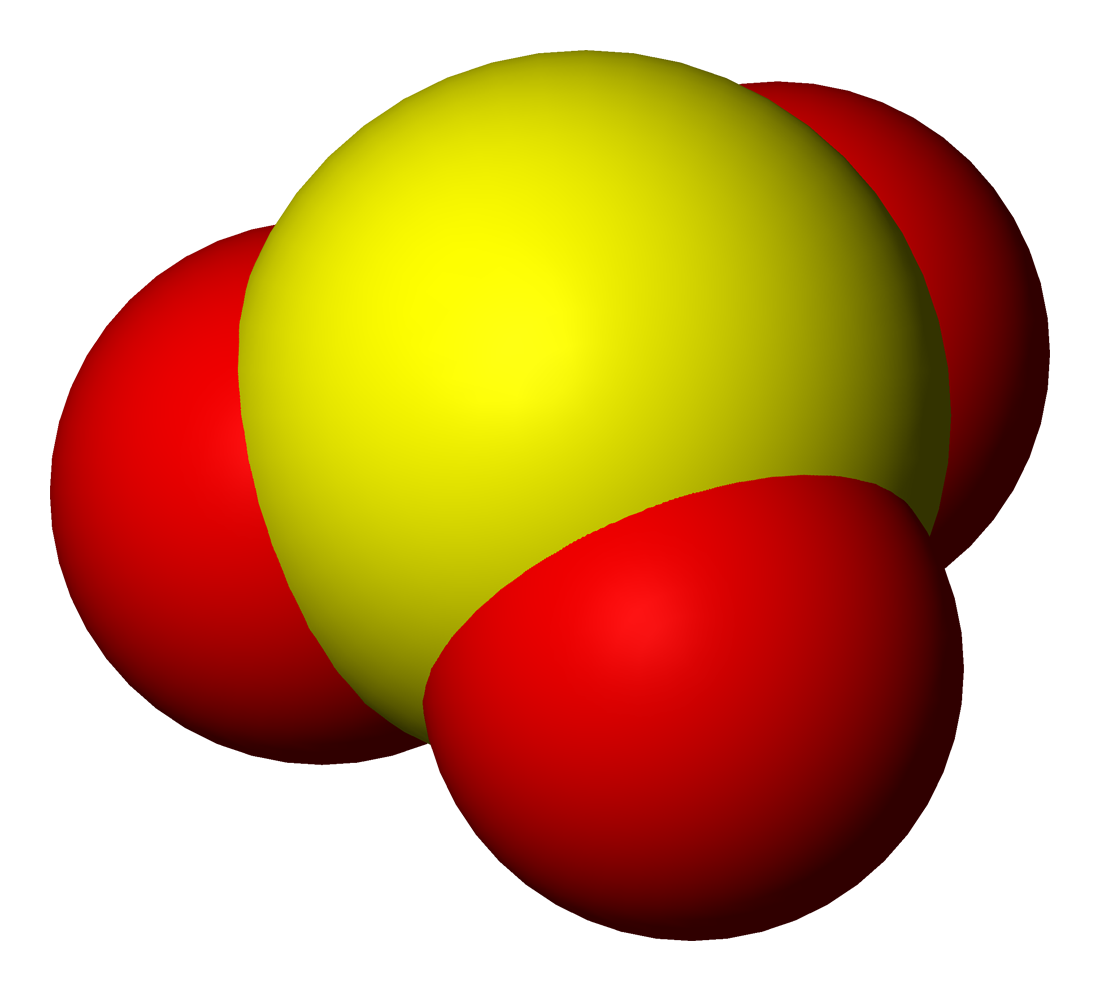
Tellurium Dioxide
Tellurium dioxide (TeO2) is a solid oxide of tellurium. It is encountered in two different forms, the yellow orthorhombic mineral tellurite, β-TeO2, and the synthetic, colourless tetragonal (paratellurite), α-TeO2. Most of the information regarding reaction chemistry has been obtained in studies involving paratellurite, α-TeO2.W.R.McWhinnie (1995) ''Tellurium - Inorganic chemistry'' Encyclopedia of Inorganic Chemistry Ed. R. Bruce King (1994) John Wiley & Sons Preparation Paratellurite, α-TeO2, is produced by reacting tellurium with O2: :Te + O2 → TeO2 An alternative preparation is to dehydrate tellurous acid, H2TeO3, or to thermally decompose basic tellurium nitrate, Te2O4·HNO3, above 400 °C. Physical properties The longitudinal speed of sound in Tellurium dioxide is at around room temperature. Chemical properties TeO2 is barely soluble in water and soluble in strong acids and alkali metal hydroxides. It is an amphoteric substance and therefore can act bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurous Acid
Tellurous acid is an inorganic compound with the formula H2TeO3. It is the oxoacid of tellurium(IV). This compound is not well characterized. An alternative way of writing its formula is (HO)2TeO. In principle, tellurous acid would form by treatment of tellurium dioxide with water, that is by hydrolysis. The related conjugate base is well known in the form of several salts such as potassium hydrogen tellurite, KHTeO3. Properties In contrast to the analogous compound selenous acid Selenous acid (or selenious acid) is the chemical compound with the formula . Structurally, it is more accurately described by . It is the principal oxoacid of selenium; the other being selenic acid. Formation and properties Selenous acid is ..., tellurous acid is only metastable. Most tellurite salts contain the ion. Oxidation of its aqueous solution with hydrogen peroxide gives the tellurate ion. It is usually prepared as an aqueous solution where it acts as a weak acid. :H2TeO3 + H2O H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Tellurite
Potassium tellurite, K2TeO3, is an inorganic potassium-tellurium Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fo ... compound. It has been used as a selective growth medium in microbiology. References {{Potassium compounds Tellurites Potassium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
:Category:Tellurites
See tellurite The tellurite ion is . A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. They are typically colorless or white salts, which in some ways are comparable to sulfite. A mineral with the formula TeO2 is ... for more info. Tellurium(IV) compounds Oxygen compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetry Group
In group theory, the symmetry group of a geometric object is the group of all transformations under which the object is invariant, endowed with the group operation of composition. Such a transformation is an invertible mapping of the ambient space which takes the object to itself, and which preserves all the relevant structure of the object. A frequent notation for the symmetry group of an object ''X'' is ''G'' = Sym(''X''). For an object in a metric space, its symmetries form a subgroup of the isometry group of the ambient space. This article mainly considers symmetry groups in Euclidean geometry, but the concept may also be studied for more general types of geometric structure. Introduction We consider the "objects" possessing symmetry to be geometric figures, images, and patterns, such as a wallpaper pattern. For symmetry of physical objects, one may also take their physical composition as part of the pattern. (A pattern may be specified formally as a scalar field ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity". Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, William Nicholson and Anthony Carlisle sought to further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/ donates electrons", that "i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenous Acid
Selenous acid (or selenious acid) is the chemical compound with the formula . Structurally, it is more accurately described by . It is the principal oxoacid of selenium; the other being selenic acid. Formation and properties Selenous acid is analogous to sulfurous acid, but it is more readily isolated. Selenous acid is easily formed upon the addition of selenium dioxide to water. As a crystalline solid, the compound can be seen as pyramidal molecules that are interconnected with hydrogen bonds. In solution it is a diprotic acid: : (p''K''a = 2.62) : (p''K''a = 8.32) It is moderately oxidizing in nature, but kinetically slow. In 1 M : : (''E''o = +0.74 V) In 1 M : : (''E''o = −0.37 V) Selenous acid is hygroscopic. Uses The major use is in protecting and changing the color of steel, especially steel parts on firearms. The so-called cold-bluing process uses selenous acid, copper(II) nitrate, and nitric acid to change the color of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corynebacteria
''Corynebacterium'' () is a genus of Gram-positive bacteria and most are aerobic. They are bacilli (rod-shaped), and in some phases of life they are, more specifically, club-shaped, which inspired the genus name (''coryneform'' means "club-shaped"). They are widely distributed in nature in the microbiota of animals (including the human microbiota) and are mostly innocuous, most commonly existing in commensal relationships with their hosts. Some, such as '' C. glutamicum'', are commercially useful. Others can cause human disease, including, most notably, diphtheria, which is caused by ''C. diphtheriae''. As with various species of amicrobiota (including their relatives in the genera '' Arcanobacterium'' and ''Trueperella''), they usually are not pathogenic, but can occasionally opportunistically capitalize on atypical access to tissues (via wounds) or weakened host defenses. Taxonomy The genus ''Corynebacterium'' was created by Lehmann and Neumann in 1896 as a taxonomic gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |