|
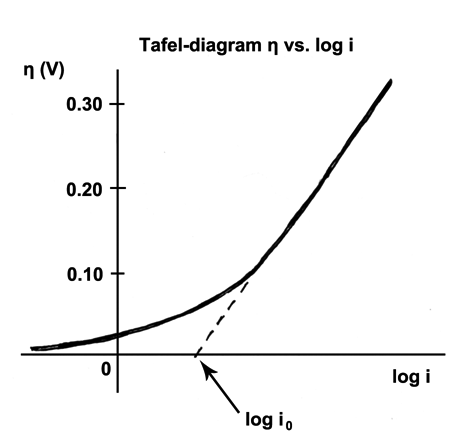
Tafel Equation
The Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification. The equation is named after Swiss chemist Julius Tafel." It describes how the electrical current through an electrode depends on the voltage difference between the electrode and the bulk electrolyte for a simple, unimolecular redox reaction ". Ox + n e^- \leftrightarrows Red Where an electrochemical reaction occurs in two half reactions on separate electrodes, the Tafel equation is applied to each electrode separately. On a single electrode the Tafel equation can be stated as: where * the plus sign under the exponent refers to an anodic reaction, and a minus sign to a cathodic reaction, *\eta : overpotential, V * A : " Tafel slope", V * i : current density, A/m2 *i_0 : " exchange current density", A/m2. A verification plus further explana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
100905 Tafel Plot Nl
1 (one, unit, unity) is a number representing a single or the only entity. 1 is also a numerical digit and represents a single unit of counting or measurement. For example, a line segment of ''unit length'' is a line segment of length 1. In conventions of sign where zero is considered neither positive nor negative, 1 is the first and smallest positive integer. It is also sometimes considered the first of the infinite sequence of natural numbers, followed by 2, although by other definitions 1 is the second natural number, following 0. The fundamental mathematical property of 1 is to be a multiplicative identity, meaning that any number multiplied by 1 equals the same number. Most if not all properties of 1 can be deduced from this. In advanced mathematics, a multiplicative identity is often denoted 1, even if it is not a number. 1 is by convention not considered a prime number; this was not universally accepted until the mid-20th century. Additionally, 1 is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Transfer Coefficient
Charge transfer coefficient, and symmetry factor (symbols ''α'' and ''β'', respectively) are two related parameters used in description of the kinetics of electrochemical reactions. They appear in the Butler–Volmer equation and related expressions. The symmetry factor and the charge transfer coefficient are dimensionless. According to an IUPAC definition, for a reaction with a single rate-determining step, the charge transfer coefficient for a cathodic reaction (the cathodic transfer coefficient, ''αc'') is defined as: :\frac = - \frac \left( \frac \right)_ The anodic transfer coefficient (''αa'') is defined by analogy: :\frac = \frac \left( \frac \right)_ where: *\nu: stoichiometric number, i.e., the number of activated complexes formed and destroyed in the overall reaction (with ''n'' electrons) * R: universal gas constant * T: absolute temperature * n: number of electrons involved in the electrode reaction * F: Faraday constant * E: electrode potential * I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faraday's Laws Of Electrolysis
Faraday's laws of electrolysis are quantitative relationships based on the electrochemical research published by Michael Faraday in 1833. First law Michael Faraday reported that the mass (m) of elements deposited at an electrode is directly proportional to the charge (Q; SI units are ampere seconds or coulombs). \begin m &\propto Q \\ \implies \frac &= Z \end Here, the constant of proportionality, Z, is called the electro-chemical equivalent (e.c.e) of the substance. Thus, the e.c.e. can be defined as the mass of the substance deposited/liberated per unit charge. Second law Faraday discovered that when the same amount of electric current is passed through different electrolytes/elements connected in series, the mass of the substance liberated/deposited at the electrodes is directly proportional to their chemical equivalent/equivalent weight (E). This turns out to be the molar mass (M) divided by the valence (v) : m \propto E : E = \frac : \implies m_1 : m_2 : m_3 : .. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faradaic Current
The faradaic current is the current generated by the reduction or oxidation of some chemical substance at an electrode. The net faradaic current is the algebraic sum of all the faradaic currents flowing through an indicator electrode or working electrode. Limiting current The limiting current in electrochemistry is the limiting value of a faradaic current that is approached as the rate of charge transfer to an electrode is increased. The limiting current can be approached, for example, by increasing the electric potential or decreasing the rate of mass transfer to the electrode. It is independent of the applied potential over a finite range, and is usually evaluated by subtracting the appropriate residual current from the measured total current. A limiting current can have the character of an adsorption, catalytic, diffusion, or kinetic current, and may include a migration current. Migration current The difference between the current that is actually obtained, at any particular v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells. Practical electrocatalysts Chloralkali process The chloralkali process is a large scale application that uses electrocatalysts. This technology supplies most of the chlorine and sodium hydroxide required by many industries. The cathode is a mixed metal oxide clad titanium anode (also called a dimensionally stable anode). Electrofluorination Many organofluorine compounds are produced by electrofluorination. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's ''voltage efficiency''. In an electrolytic cell the existence of overpotential implies that the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density (typically small) is achieved. Thermodynamics The four possible polarities of overpotentials are listed below. * An electrolytic cell's anode ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ohm's Law
Ohm's law states that the current through a conductor between two points is directly proportional to the voltage across the two points. Introducing the constant of proportionality, the resistance, one arrives at the usual mathematical equation that describes this relationship: :I = \frac, where is the current through the conductor, ''V'' is the voltage measured ''across'' the conductor and ''R'' is the resistance of the conductor. More specifically, Ohm's law states that the ''R'' in this relation is constant, independent of the current. If the resistance is not constant, the previous equation cannot be called ''Ohm's law'', but it can still be used as a definition of static/DC resistance. Ohm's law is an empirical relation which accurately describes the conductivity of the vast majority of electrically conductive materials over many orders of magnitude of current. However some materials do not obey Ohm's law; these are called non-ohmic. The law was named after the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Universal Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of subs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faraday Constant
In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of SI base units, which took effect on 20 May 2019, the Faraday constant has the exactly defined value given by the product of the elementary charge ''e'' and Avogadro constant ''N''A: : : :. Derivation The Faraday constant can be thought of as the conversion factor between the mole (used in chemistry) and the coulomb (used in physics and in practical electrical measurements), and is therefore of particular use in electrochemistry. Because 1 mole contains exactly entities, and 1 coulomb contains exactly elementary charges, the Faraday constant is given by the quotient of these two quantities: :. One common use of the Faraday constant is in electrolysis calculations. One can divide the amount of charge (the current integrated over time) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Rate Constant
In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction. For a reaction between reactants A and B to form product C the reaction rate is often found to have the form: r = k(T) mathrmm mathrm Here ''k''(''T'') is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reaction mechanism and can be determined experimentally. Elementary steps For an elementary step, there ''is'' a relationship between stoichiometry and rate law, as determined b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nernst Equation
In electrochemistry, the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction ( half-cell or full cell reaction) from the standard electrode potential, absolute temperature, the number of electrons involved in the redox reaction, and activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation respectively. It was named after Walther Nernst, a German physical chemist who formulated the equation. Expression General form with chemical activities When an oxidizer () accepts a number ''z'' of electrons () to be converted in its reduced form (), the half-reaction is expressed as: : + ''z'' → The reaction quotient ('), also often called the ion activity product (''IAP''), is the ratio between the chemical activities (''a'') of the reduced form (the reductant, ) and the oxidized form (the oxidant, ). The chemical activity of a dissolved species co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)
