|
TREM2
Triggering receptor expressed on myeloid cells 2 (TREM2) is a protein that in humans is encoded by the ''TREM2'' gene. TREM2 is expressed on macrophages, immature monocyte-derived dendritic cells, osteoclasts, and microglia, which are immune cells in the central nervous system. In the liver, TREM2 is expressed by several cell types, including macrophages, that respond to injury. In the intestine, TREM2 is expressed by myeloid-derived dendritic cells and macrophage. TREM2 is overexpressed in many tumor types and has anti-inflammatory activities. It might therefore be a good therapeutic target. Gene The TREM2' gene lies on the sixth chromosome in humans, specifically in location 6p21.1. The gene has 5 coding exon regions. Alternative splicing of the ''TREM2'' mRNA transcript leads to different isoforms of the protein being produced upon translation. Specifically, ''TREM2'' mRNA has 3 different isoforms containing 3 consistent exons, and 2 that vary between the isoforms. ''TREM2'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADAM10
A Disintegrin and metalloproteinase domain-containing protein 10, also known as ADAM10 or CDw156 or CD156c is a protein that in humans is encoded by the ''ADAM10'' gene. Function Members of the ADAM family are cell surface proteins with a unique structure, possessing both potential adhesion and protease domains. Sheddase, a generic name for the ADAM metallopeptidase, functions primarily to cleave membrane proteins at the cellular surface. Once cleaved, the sheddases release soluble ectodomains with an altered location and function. Although a single sheddase may “shed” a variety of substances, multiple sheddases can cleave the same substrate resulting in different consequences. This gene encodes an ADAM family member that cleaves many proteins including TNF-alpha and E-cadherin. ADAM10 (EC#: 3.4.24.81) is a sheddase, and has a broad specificity for peptide hydrolysis reactions. ADAM10 cleaves ephrin, within the ephrin/eph complex, formed between two cell surfaces. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DAP12
TYRO protein tyrosine kinase-binding protein is an adapter protein that in humans is encoded by the ''TYROBP'' gene. Function This gene encodes a transmembrane signaling polypeptide which contains an immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic domain. The encoded protein may associate with the killer cell immunoglobulin-like receptor (KIR) family of membrane glycoproteins and may act as an activating signal transduction element. This protein may bind zeta-chain associated protein kinase 70 kDa (ZAP-70) and spleen tyrosine kinase (SYK) and play a role in signal transduction, bone modeling, brain myelination, and inflammation. Mutations within this gene have been associated with polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL), also known as Nasu-Hakola disease. Its putative receptor, triggering receptor expressed on myeloid cells 2 (TREM2), also causes PLOSL. Two alternative transcript variants encoding distinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IFNGR1
Interferon gamma receptor 1 (IFNGR1) also known as CD119 (Cluster of Differentiation 119), is a protein that in humans is encoded by the ''IFNGR1'' gene. Function The gene ''IFNGR1'' encodes IFN-γR1, which is the ligand-binding chain (alpha) of the heterodimeric gamma interferon receptor, which is found on macrophages. IFNGR2, encodes IFN-γR2, the non-ligand-binding partner of the heterodimeric receptor. Interactions Interferon gamma receptor 1 has been shown to interact with Interferon-gamma. Mutations Mutations in the ''IFNGR1'' gene can lead to extreme susceptibility to Mycobacterial infections. All known mutations and common variations in the IFNGR1 are present in the IFNGR1 mutation database. See also * Cluster of differentiation * Interferon-gamma receptor The interferon-gamma receptor (IFNGR) protein complex is the heterodimer of two chains: IFNGR1 and IFNGR2. It binds interferon-γ, the sole member of interferon type II. Structure and function The hu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
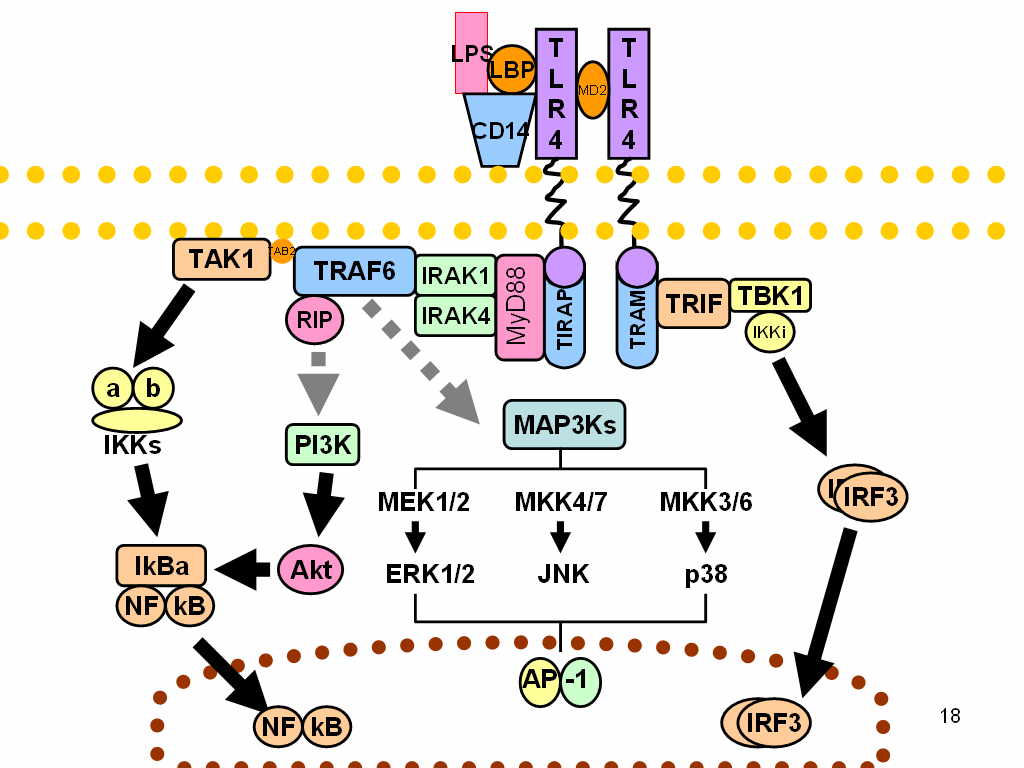
TLR4
Toll-like receptor 4 is a protein that in humans is encoded by the ''TLR4'' gene. TLR4 is a transmembrane protein, member of the toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. Its activation leads to an intracellular signaling pathway NF-κB and inflammatory cytokine production which is responsible for activating the innate immune system. TLR4 expressing cells are myeloid (erythrocytes, granulocytes, macrophages) rather than lymphoid (T-cells, B-cells, NK cells). Most myeloid cells also express high levels of CD14, which facilitates activation of TLR4 by LPS. It is most well known for recognizing lipopolysaccharide (LPS), a component present in many Gram-negative bacteria (e.g. ''Neisseria'' spp.) and selected Gram-positive bacteria. Its ligands also include several viral proteins, polysaccharide, and a variety of endogenous proteins such as low-density lipoprotein, beta-defensins, and heat shock protein. Palmitic acid and lauric acid a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toll-like Receptor
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are Bitopic protein, single-pass membrane-spanning Receptor (biochemistry), receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in Intracellular receptor, intracellular Vesicle (biology and chemistry), vesicles (because they are sensors of nucleic acids). TLRs received their name from their similarity to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
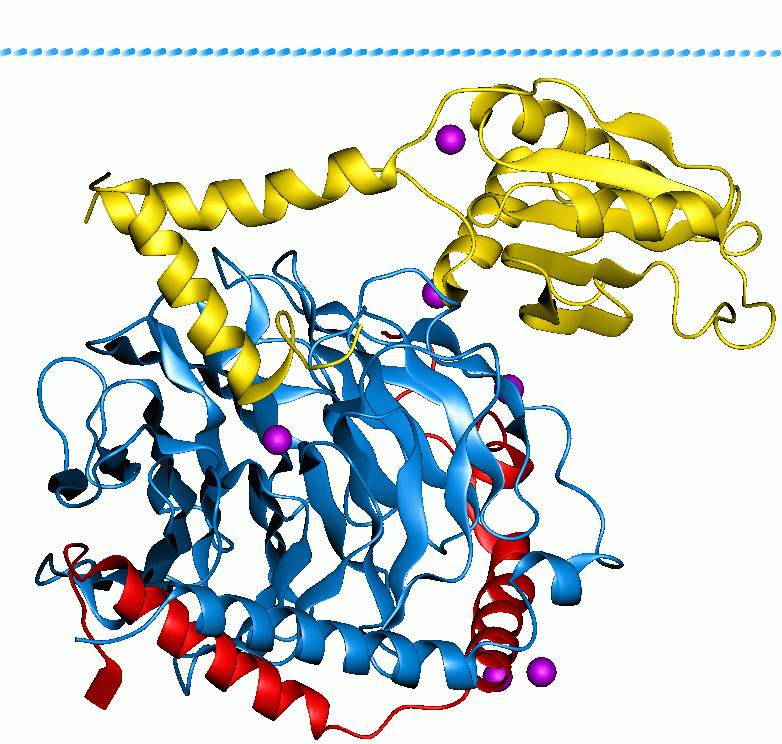
CD14
CD14 (cluster of differentiation 14) is a human protein made mostly by macrophages as part of the innate immune system. It helps to detect bacteria in the body by binding lipopolysaccharide (LPS), a pathogen-associated molecular pattern (PAMP). CD14 exists in two forms, one anchored to the membrane by a glycosylphosphatidylinositol (GPI) tail (mCD14), the other a soluble form (sCD14). Soluble CD14 either appears after shedding of mCD14 (48 kDa) or is directly secreted from intracellular vesicles (56 kDa). The x-ray crystal structure of human CD14 reveals a monomeric, bent solenoid structure containing a hydrophobic amino-terminal pocket. CD14 was the first described pattern recognition receptor. Function CD14 acts as a co-receptor (along with the Toll-like receptor TLR 4 and MD-2) for the detection of bacterial lipopolysaccharide (LPS). CD14 can bind LPS only in the presence of lipopolysaccharide-binding protein (LBP). Although LPS is considered its main ligand, CD14 also rec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FPR1
Formyl peptide receptor 1 (FPR1, FPR1 receptor, fMet-Leu-Phe receptor 1, FMLP receptor 1, or N-formylmethionyl-leucyl-phenylalanine receptor 1) is a cell surface receptor protein that in humans is encoded by the ''formyl peptide receptor 1'' (''FPR1'') gene. This gene encodes a G protein-coupled receptor cell surface protein that binds and is activated by N-Formylmethionine-containing oligopeptides, particularly N-Formylmethionine-leucyl-phenylalanine (FMLP). FPR1 is prominently expressed by mammalian phagocytic and blood leukocyte cells where it functions to mediate these cells' responses to the N-formylmethionine-containing oligopeptides which are released by invading microorganisms and injured tissues. FPR1 directs these cells to sites of invading pathogens or disrupted tissues and then stimulates these cells to kill the pathogens or to remove tissue debris; as such, it is an important component of the innate immune system that operates in host defense and damage control. Hum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Granulocyte
Granulocytes are cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear. They have varying shapes (morphology) of the nucleus (segmented, irregular; often lobed into three segments); and are referred to as polymorphonuclear leukocytes (PMN, PML, or PMNL). In common terms, ''polymorphonuclear granulocyte'' refers specifically to "neutrophil granulocytes", the most abundant of the granulocytes; the other types (eosinophils, basophils, and mast cells) have varying morphology. Granulocytes are produced via granulopoiesis in the bone marrow. Types There are four types of granulocytes (full name polymorphonuclear granulocytes): * Basophils * Eosinophils * Neutrophils * Mast cells Except for the mast cells, their names are derived from their staining characteristics; for example, the most abundant granulocyte is the neut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
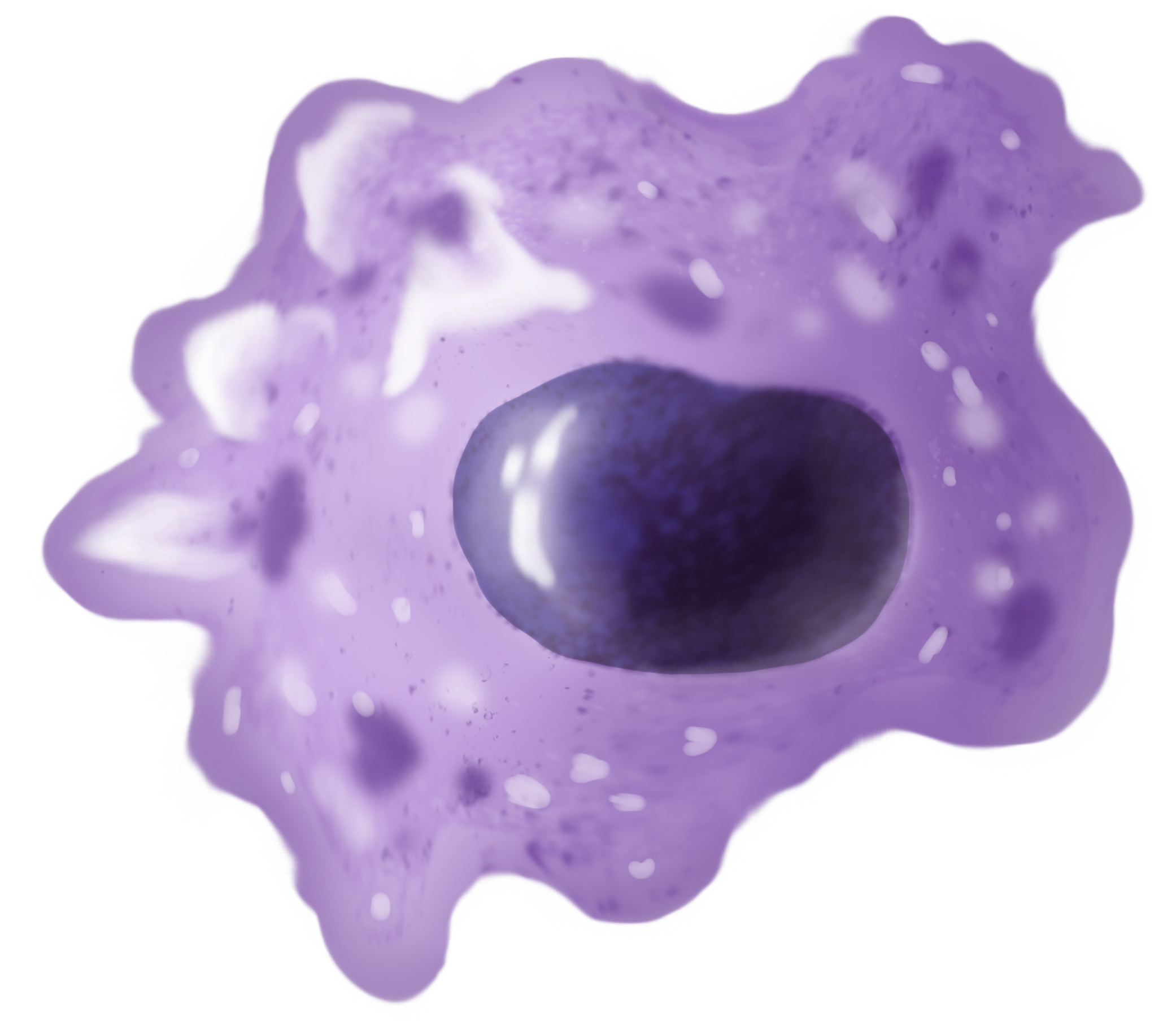
Macrophages
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. The process is called phagocytosis, which acts to defend the host against infection and injury. These large phagocytes are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microglia, and others), but all are part of the mononuclear phagocyte system. Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity) and also help initiate specific defense mechanisms (adaptive immunity) by recruiting other immun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelocyte
A myelocyte is a young cell of the granulocytic series, occurring normally in bone marrow (can be found in circulating blood when caused by certain diseases). Structure When stained with the usual dyes, the cytoplasm is distinctly basophilic and relatively more abundant than in myeloblasts or promyelocytes, even though myelocytes are smaller cells. Numerous cytoplasmic granules are present in the more mature forms of myelocytes. Neutrophilic and eosinophilic granules are peroxidase-positive, while basophilic granules are not. The nuclear chromatin is coarser than that observed in a promyelocyte, but it is relatively faintly stained and lacks a well-defined membrane. The nucleus is fairly regular in contour (not indented), and seems to be 'buried' beneath the numerous cytoplasmic granules. (If the nucleus were indented, it would likely be a metamyelocyte.) Measurement There is an internationally agreed method of counting blasts, with results from M1 upwards. Development ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |