|
Solder Fatigue
Solder fatigue is the mechanical degradation of solder due to deformation under cyclic loading. This can often occur at stress levels below the yield stress of solder as a result of repeated temperature fluctuations, mechanical vibrations, or mechanical loads. Techniques to evaluate solder fatigue behavior include finite element analysis and semi-analytical closed-form equations. Overview Solder is a metal alloy used to form electrical, thermal, and mechanical interconnections between the component and printed circuit board (PCB) substrate in an electronic assembly. Although other forms of cyclic loading are known to cause solder fatigue, it has been estimated that the largest portion of electronic failures are thermomechanically driven due to temperature cycling. Under thermal cycling, stresses are generated in the solder due to coefficient of thermal expansion (CTE) mismatches. This causes the solder joints to experience non-recoverable deformation via creep and plasticity tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solder
Solder (; NA: ) is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics. Soft solder typically has a melting point range of , and is commonly used in electronics, plumbing, and sheet metal work. Alloys that melt between are the most commonly used. Soldering performed using alloys with a melting point above is called "hard soldering", "silver soldering", or brazing. In specific proportions, some alloys are eutectic — that is, the alloy's melting point is the lowest possible for a mixture of those components, and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Restriction Of Hazardous Substances Directive
The Restriction of Hazardous Substances Directive 2002/95/EC (RoHS 1), short for Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, was adopted in February 2003 by the European Union. The initiative was to prevent an overabundance of chemicals in electronics. Thus, as a result electronics were restricted. The RoHS 1 directive took effect on 1 July 2006, and is required to be enforced and became a law in each member state. This directive restricts (with exceptions) the use of ten hazardous materials in the manufacture of various types of electronic and electrical equipment. In addition to the exceptions, there are exclusions for products such as solar panels. It is closely linked with the Waste Electrical and Electronic Equipment Directive (WEEE) 2002/96/EC (now superseded) which sets collection, recycling and recovery targets for electrical goods and is part of a legislative initiative to solve the problem of h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low Cycle Fatigue
Low cycle fatigue (LCF) has two fundamental characteristics: plastic deformation in each cycle; and low cycle phenomenon, in which the materials have finite endurance for this type of load. The term ''cycle'' refers to repeated applications of stress that lead to eventual fatigue and failure; ''low-cycle'' pertains to a long period between applications. Study in fatigue has been focusing on mainly two fields: size design in aeronautics and energy production using advanced calculation methods. The LCF result allows us to study the behavior of the material in greater depth to better understand the complex mechanical and metallurgical phenomena (crack propagation, work softening, strain concentration, work hardening, etc.). History Common factors that have been attributed to low-cycle fatigue (LCF) are high stress levels and a low number of cycles to failure. Many studies have been carried out, particularly in the last 50 years on metals and the relationship between temperature, st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homologous Temperature
Homologous temperature expresses the thermodynamic temperature of a material as a fraction of the thermodynamic temperature of its melting point (i.e. using the Kelvin scale): T_H = \frac For example, the homologous temperature of lead at room temperature (25 °C) is approximately 0.50 (TH = T/Tmp = 298 K/601 K = 0.50). Significance of the homologous temperature The homologous temperature of a substance is useful for determining the rate of steady state creep (diffusion dependent deformation). A higher homologous temperature results in an exponentially higher rate of diffusion dependent deformation. Additionally, for a given fixed homologous temperature, two materials with different melting points would have similar diffusion-dependent deformation behaviour. For example, solder (Tmp = 456 K) at 115 °C would have comparable mechanical properties to copper (Tmp = 1358 K) at 881 °C, because they would both be at 0.85Tmp despite being at diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscoplastic
Viscoplasticity is a theory in continuum mechanics that describes the rate-dependent inelastic behavior of solids. Rate-dependence in this context means that the deformation of the material depends on the rate at which loads are applied. The inelastic behavior that is the subject of viscoplasticity is plastic deformation which means that the material undergoes unrecoverable deformations when a load level is reached. Rate-dependent plasticity is important for transient plasticity calculations. The main difference between rate-independent plastic and viscoplastic material models is that the latter exhibit not only permanent deformations after the application of loads but continue to undergo a creep flow as a function of time under the influence of the applied load. The elastic response of viscoplastic materials can be represented in one-dimension by Hookean spring elements. Rate-dependence can be represented by nonlinear dashpot elements in a manner similar to viscoelasticity. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crack Propagation
Fracture mechanics is the field of mechanics concerned with the study of the propagation of cracks in materials. It uses methods of analytical solid mechanics to calculate the driving force on a crack and those of experimental solid mechanics to characterize the material's resistance to fracture. Theoretically, the stress ahead of a sharp crack tip becomes infinite and cannot be used to describe the state around a crack. Fracture mechanics is used to characterise the loads on a crack, typically using a single parameter to describe the complete loading state at the crack tip. A number of different parameters have been developed. When the plastic zone at the tip of the crack is small relative to the crack length the stress state at the crack tip is the result of elastic forces within the material and is termed linear elastic fracture mechanics (LEFM) and can be characterised using the stress intensity factor K. Although the load on a crack can be arbitrary, in 1957 G. Irwin found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to slide over each other at low stress levels and is known as ''glide'' or slip. The crystalline order is restored on either side of a ''glide dislocation'' but the atoms on one side have moved by one position. The crystalline order is not fully restored with a ''partial dislocation''. A dislocation defines the boundary between ''slipped'' and ''unslipped'' regions of material and as a result, must either form a complete loop, intersect other dislocations or defects, or extend to the edges of the crystal. A dislocation can be characterised by the distance and direction of movement it causes to atoms which is defined by the Burgers vector. Plastic deformation of a material occurs by the creation and movement of many dislocations. The number and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
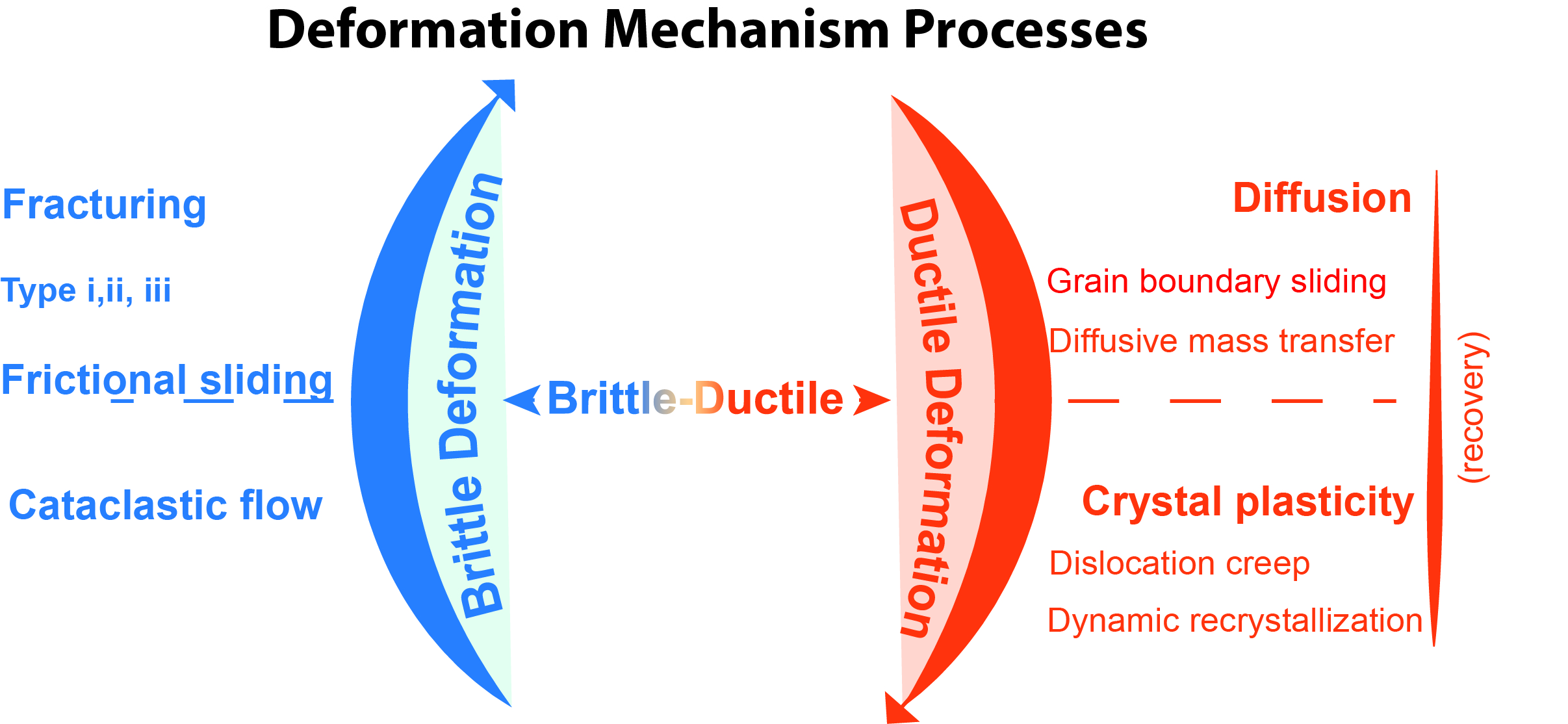
Deformation Mechanism
A deformation mechanism, in geology, is a process occurring at a microscopic scale that is responsible for changes in a material's internal structure, shape and volume. The process involves planar discontinuity and/or displacement of atoms from their original position within a crystal lattice structure. These small changes are preserved in various microstructures of materials such as rocks, metals and plastics, and can be studied in depth using optical or digital microscopy. Processes Deformation mechanisms are commonly characterized as brittle, ductile, and brittle-ductile. The driving mechanism responsible is an interplay between internal (e.g. composition, grain size and lattice-preferred orientation) and external (e.g. temperature and fluid pressure) factors. These mechanisms produce a range of micro-structures studied in rocks to constrain the conditions, rheology, dynamics, and motions of tectonic events. More than one mechanism may be active under a given set of conditions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermetallics
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds. Although the term "intermetallic compounds", as it applies to solid phases, has been in use for many years, its introduction was regretted, for example by Hume-Rothery in 1955. Definitions Research definition Schulze in 1967 defined intermetallic compounds as ''solid phases containing two or more metallic elements, with optionally one or more non-metallic elements, whose crystal structure differs from that of the other constituents''. Under this definition, the following are included: #Electron (or Hume-Rothery) compounds #Size packing phases. e.g. Lav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |