|
Simmons–Smith Reaction
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene (or alkyne) to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific. : Thus, cyclohexene, diiodomethane, and a zinc-copper couple (as iodomethylzinc iodide, ICH2ZnI) yield norcarane (bicyclo .1.0eptane). : The Simmons–Smith reaction is generally preferred over other methods of cyclopropanation, however it can be expensive due to the high cost of diiodomethane. Modifications involving cheaper alternatives have been developed, such as dibromomethane or diazomethane and zinc iodide. The reactivity of the system can also be increased by using the Furukawa modification, exchanging the zinc‑copper cou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Howard Ensign Simmons, Jr
Howard is an English-language given name originating from Old French Huard (or Houard) from a Germanic source similar to Old High German ''*Hugihard'' "heart-brave", or ''*Hoh-ward'', literally "high defender; chief guardian". It is also probably in some cases a confusion with the Old Norse cognate ''Haward'' (''Hávarðr''), which means "high guard" and as a surname also with the unrelated Hayward. In some rare cases it is from the Old English ''eowu hierde'' "ewe herd". In Anglo-Norman the French digram ''-ou-'' was often rendered as ''-ow-'' such as ''tour'' → ''tower'', ''flour'' (western variant form of ''fleur'') → ''flower'', etc. (with svarabakhti). A diminutive is "Howie" and its shortened form is "Ward" (most common in the 19th century). Between 1900 and 1960, Howard ranked in the U.S. Top 200; between 1960 and 1990, it ranked in the U.S. Top 400; between 1990 and 2004, it ranked in the U.S. Top 600. People with the given name Howard or its variants include: Given ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norcarane
Norcarane, or bicyclo .1.0eptane, is a colorless liquid. It is an organic compound prepared using the Simmons–Smith reaction, by the action of diiodomethane and a zinc-copper couple on cyclohexene in diethyl ether Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li .... : References Hydrocarbons Cyclopropanes Cyclohexanes Bicycloalkanes {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
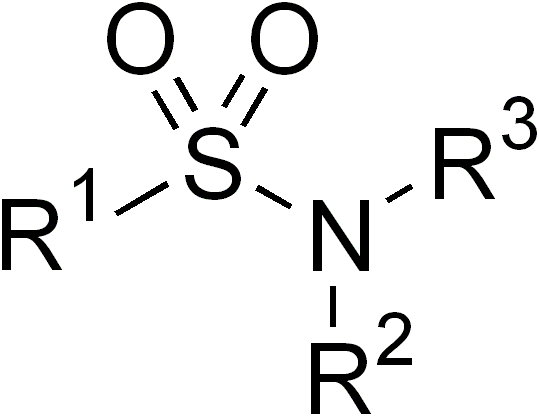
Sulfonamide (chemistry)
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamyl Alcohol
Cinnamyl alcohol or styron is an organic compound that is found in esterified form in storax, Balsam of Peru, and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the hydrolysis of storax. Cinnamyl alcohol has a distinctive odour described as "sweet, balsam, hyacinth, spicy, green, powdery, cinnamic" and is used in perfumery and as a deodorant. Cinnamyl alcohol is naturally occurrent only in small amount, so its industrial demand is usually fulfilled by chemical synthesis starting from cinnamaldehyde. Properties The compound is a solid at room temperature, forming colourless crystals that melt upon gentle heating. As is typical of most higher-molecular weight alcohols, it is sparingly soluble in water at room temperature, but highly soluble in most common organic solvents. Safety Cinnamyl alcohol has been found to have a sensitising effect on some people [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 2.415. Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Publications established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisoxazoline Ligand
In chemistry, bis(oxazoline) ligands (often abbreviated BOX ligands) are a class of privileged chiral ligands containing two oxazoline rings. They are typically C2‑symmetric and exist in a wide variety of forms; with structures based around CH2 or pyridine linkers being particularly common (often generalised BOX and PyBOX respectively). The coordination complexes of bis(oxazoline) ligands are used in asymmetric catalysis. These ligands are examples of C2-symmetric ligands. Synthesis The synthesis of oxazoline rings is well established and in general proceeded via the cyclisation of a 2‑amino alcohol with any of a number of suitable functional groups. In the case of bis(oxazoline)s, synthesis is most conveniently achieved by using bi-functional starting materials; as this allows both rings to be produced at once. Of the materials suitable, dicarboxylic or di nitrile compounds are the most commonly available and hence the majority bis(oxazoline) ligands are produced from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazo
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N–. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds (R2C=N2) should not be confused with azo compounds of the type R-N=N-R or with diazonium compounds of the type R-N2+. Structure The electronic structure of diazo compounds is characterized by π electron density delocalized over the α-carbon and two nitrogen atoms, along with an orthogonal π system with electron density delocalized over only the terminal nitrogen atoms. Because all octet rule-satisfying resonance forms of diazo compounds have formal charges, they are members of a class of compounds known as 1,3-dipoles. Some of the most stable diazo compounds are α-diazo-β-diketones an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Reaction
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric ( enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylzinc
Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a solution in hexanes, heptane, or toluene, or as a pure liquid. Synthesis Edward Frankland first reported the compound in 1848 from zinc and ethyl iodide, the first organozinc compound discovered. He improved the synthesis by using diethyl mercury as starting material. The contemporary synthesis consists of the reaction of a 1:1 mixture of ethyl iodide and ethyl bromide with a zinc-copper couple, a source of reactive zinc. Structure The compound crystallizes in a tetragonal body-centered unit cell of space group symmetry I41md. In the solid-state diethylzinc shows nearly linear Zn centres. The Zn-C bonds measure 194.8(5) pm, while the C-Zn-C angle is slightly bent with 176.2(4)°. The structure of the gas-phase shows a very similar Zn-C d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Iodide
Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application. Preparation It can be prepared by the direct reaction of zinc and iodine in water or in refluxing ether. or by treating zinc with iodine in aqueous solution: : Zn + I2 → ZnI2 Structure as solid, gas, and in solution The structure of solid ZnI2 is unusual relative to the dichloride. While zinc centers are tetrahedrally coordinated, as in ZnCl2, groups of four of these tetrahedra share three vertices to form “super-tetrahedra” of composition , which are linked by their vertices to form a three-dimensional structure. These "super-tetrahedra" are similar to the P4O10 structure. Molecular ZnI2 is linear as predicted by VSEPR theory with a Zn-I bond length of 238 pm. In aqueous solution the following have been detected: Zn(H2O)62+, nI(H2O)5sup>+, tetrahedral ZnI2(H2O)2, Zn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |