|
Second Law Efficiency
Exergy efficiency (also known as the second-law efficiency or rational efficiency) computes the effectiveness of a system relative to its performance in reversible conditions. It is defined as the ratio of the thermal efficiency of an actual system compared to an idealized or reversible version of the system for heat engines. It can also be described as the ratio of the useful work output of the system to the reversible work output for work-consuming systems. For refrigerators and heat pumps, it is the ratio of the actual COP and reversible COP. Motivation The reason the second-law efficiency is needed is because the first-law efficiencies fail to take into account an idealized version of the system for comparison. Using first-law efficiencies alone, can lead one to believe a system is more efficient than it is in reality. So, the second-law efficiencies are needed to gain a more realistic picture of a system's effectiveness. From the second law of thermodynamics it can be demons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc. For a heat engine, thermal efficiency is the ratio of the net work output to the heat input; in the case of a heat pump, thermal efficiency (known as the ''coefficient of performance'') is the ratio of net heat output (for heating), or the net heat removed (for cooling) to the energy input (external work). The efficiency of a heat engine is fractional as the output is always less than the input while the COP of a heat pump is more than 1. These values are further restricted by the Carnot theorem. Overview In general, energy conversion efficiency is the ratio between the useful output of a device and the input, in energy terms. For thermal efficiency, the input, Q_, to the device is heat, or the heat-content of a fuel that is consumed. The de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag. In general, an engine is any machine that converts energy to mechanical work. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refrigerator
A refrigerator, colloquially fridge, is a commercial and home appliance consisting of a thermally insulated compartment and a heat pump (mechanical, electronic or chemical) that transfers heat from its inside to its external environment so that its inside is cooled to a temperature below the room temperature. Refrigeration is an essential food storage technique around the world. The lower temperature lowers the reproduction rate of bacteria, so the refrigerator reduces the rate of spoilage. A refrigerator maintains a temperature a few degrees above the freezing point of water. The optimal temperature range for perishable food storage is .Keep your fridge-freezer clean and ice-free ''BBC''. 30 April 2008 A similar device that maintains a temperature below the freezing point of water is called a freezer. The refrigerator replaced the icebox, which had been a common household appliance for almost a century and a half. The United States Food and Drug Administration recommends t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing heat from the enclosed space and rejecting it outside. Units that only provide cooling are called air conditioners. When in heating mode, a refrigerant at outside temperature is being compressed. As a result, the refrigerant becomes hot. This thermal energy can be transferred to an indoor unit. After being moved outdoors again, the refrigerant is decompressed — evaporated. It has lost some of its thermal energy and returns colder than the environment. It can now take up the surrounding energy from the air or from the ground before the process repeats. Compressors, fans, and pumps run with electric energy. Common types are air-source heat pumps, ground-source heat pumps, water-source heat pumps and exhaust air heat pumps. They ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coefficient Of Performance
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy (power) consumption and thus lower operating costs. The COP usually exceeds 1, especially in heat pumps, because, instead of just converting work to heat (which, if 100% efficient, would be a COP of 1), it pumps additional heat from a heat source to where the heat is required. Most air conditioners have a COP of 2.3 to 3.5. Less work is required to move heat than for conversion into heat, and because of this, heat pumps, air conditioners and refrigeration systems can have a coefficient of performance greater than one. However, this does not mean that they are more than 100% efficient, in other words, no heat engine can have a thermal efficiency of 100% or greater. For complete systems, COP calculations should include energy consumption o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy, adapted for thermodynamic processes. It distinguishes in principle two forms of energy transfer, heat and thermodynamic work for a system of a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of energies in the system. The law of conservation of energy states that the total energy of any isolated system, which cannot exchange energy or matter, is constant. Energy can be transformed from one form to another, but can be neither created nor destroyed. The first law for a thermodynamic process is often formulated asThe sign convention (Q is heat supplied ''to'' the system but W is work done ''by'' the system) is that of Rudolf Clausius (Equation IIa on page 384 of Clausius, R. (1850)), and it is followed below. :\Delta U = Q - W, where \Delta U denotes the change in the internal energy of a closed syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
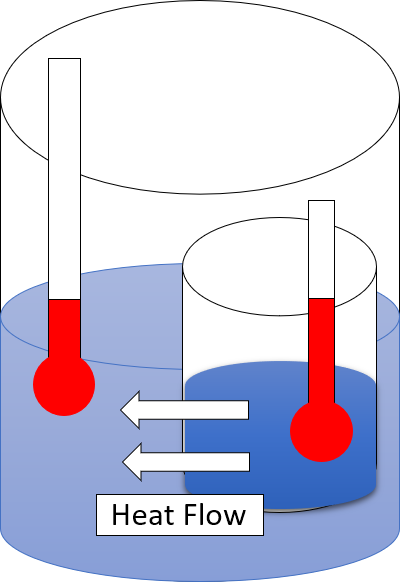
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot decr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exergy
In thermodynamics, the exergy of a system is the maximum useful work possible during a process that brings the system into equilibrium with a heat reservoir, reaching maximum entropy. When the surroundings are the reservoir, exergy is the potential of a system to cause a change as it achieves equilibrium with its environment. Exergy is the energy that is available to be used. After the system and surroundings reach equilibrium, the exergy is zero. Determining exergy was also the first goal of thermodynamics. The term "exergy" was coined in 1956 by Zoran Rant (1904–1972) by using the Greek '' ex'' and ''ergon'' meaning "from work", but the concept had been earlier developed by J Willard Gibbs (the namesake of Gibbs free energy) in 1873. Energy is neither created nor destroyed during a process. Energy changes from one form to another (''see First Law of Thermodynamics''). In contrast, exergy is always destroyed when a process is irreversible, for example loss of heat to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Function
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces. The Gibbs energy is the thermodynamic potential that is minimi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Heat Engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates. A heat engine acts by transferring energy from a warm region to a cool region of space and, in the process, converting some of that energy to mechanical work. The cycle may also be reversed. The system may be worked upon by an external force, and in the process, it can transfer thermal energy from a cooler system to a warmer one, thereby acting as a refrigerator or heat pump rather than a heat engine. Every th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Grove in 1838. The first commercial use of fuel cells came more than a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for satellites and space capsules. Since then, fuel cells have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maximum Power Principle
The maximum power principle or Lotka's principle has been proposed as the fourth principle of energetics in open system thermodynamics, where an example of an open system is a biological cell. According to Howard T. Odum, "The maximum power principle can be stated: During self-organization, system designs develop and prevail that maximize power intake, energy transformation, and those uses that reinforce production and efficiency."H.T. Odum 1995, p. 311 History Chen (2006) has located the origin of the statement of maximum power as a formal principle in a tentative proposal by Alfred J. Lotka (1922a, b). Lotka's statement sought to explain the Darwinian notion of evolution with reference to a physical principle. Lotka's work was subsequently developed by the systems ecologist Howard T. Odum in collaboration with the chemical engineer Richard C. Pinkerton, and later advanced by the engineer Myron Tribus. While Lotka's work may have been a first attempt to formalise evolu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |