|
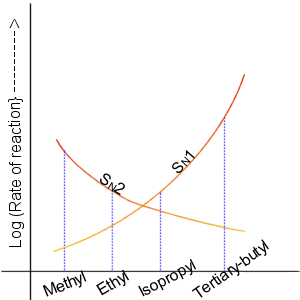
SN2
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed in a concerted way, i.e., in one step. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular, bi-molecular mechanism, which means both the reacting species are involved in the rate-determining step, rate-determining step. The other major type of nucleophilic substitution is the SN1 reaction, SN1, but many other more specialized mechanisms describe substitution reactions. The SN2 reaction can be considered as an analogue of the associative substitution in the field of inorganic chemistry. Reaction mechanism The reaction most often occurs at an aliphatic orbital hybridisation, sp3 carbon center with an electronegative, stable leaving group attached to it (often denoted X), which is frequently a halide atom. The brea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walden Inversion
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in an SN2 reaction, Walden inversion occurs at a tetrahedral carbon atom. It can be visualized by imagining an umbrella turned inside-out in a gale. In the Walden inversion, the backside attack by the nucleophile in an SN2 reaction gives rise to a product whose configuration is opposite to the reactant. Therefore, during SN2 reaction, 100% inversion of product takes place. This is known as Walden inversion. It was first observed by chemist Paul Walden in 1896. He was able to convert one enantiomer of a chemical compound into the other enantiomer and back again in a so-called Walden cycle which went like this: (+) chlorosuccinic acid (1 in the illustration) was converted to (+) malic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN1 Reaction
The SN1 reaction is a substitution reaction in organic chemistry, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction occurs. In inorganic chemistry, the SN1 reaction is often known as the ''dissociative substitution''. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Associative Substitution
Associative substitution describes a pathway by which compounds interchange ligands. The terminology is typically applied to organometallic and coordination complexes, but resembles the Sn2 mechanism in organic chemistry. The opposite pathway is dissociative substitution, being analogous to the Sn1 pathway. Intermediate pathways exist between the pure associative and pure dissociative pathways, these are called interchange mechanisms. Associative pathways are characterized by binding of the attacking nucleophile to give a discrete, detectable intermediate followed by loss of another ligand. Complexes that undergo associative substitution are either coordinatively unsaturated or contain a ligand that can change its bonding to the metal, e.g. change in hapticity or bending of a nitrogen oxide ligand (NO). In homogeneous catalysis, the associative pathway is desirable because the binding event, and hence the selectivity of the reaction, depends not only on the nature of the meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coefficients of reactants in the elementary reaction with effective collision ( sufficient energy) and correct orientation. Depending on how many molecules come together, a reaction can be unimolecular, bimolecular or even trimolecular. The kinetic order of any elementary reaction or reaction step is ''equal'' to its molecularity, and the rate equation of an elementary reaction can therefore be determined by inspection, from the molecularity. The kinetic order of a complex (multistep) reaction, however, is not necessarily equal to the number of molecules involved. The concept of molecularity is only useful to describe elementary reactions or steps. Unimolecular reactions In a unimolecular reaction, a single molecule rearranges atoms, forming ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Bromide
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor. Preparation The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene: :H2C=CH2 + HBr → H3C-CH2Br Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated ''in situ''. Uses In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon. In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters, carbanions to ethylated derivatives, thioure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question ( dynamic stereochemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm. Natural occurrence Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper. Formation and react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Chloride
Chloroethane, commonly known as ethyl chloride, is a chemical compound with chemical formula CH3CH2Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor. Production Chloroethane is produced by hydrochlorination of ethylene: :C2H4 + HCl → C2H5Cl At various times in the past, chloroethane has also been produced from ethanol and hydrochloric acid, from ethane and chlorine, or from ethanol and phosphorus trichloride, but these routes are no longer economical. Some chloroethane is generated as a byproduct of polyvinyl chloride production. Uses Chloroethane is an inexpensive ethylating agent. It reacts with aluminium metal to give ethylaluminium sesquichloride, a precursor to polymers and other useful organoaluminium compounds. Chloroethane is used to convert cellulose to ethylcellulose, a thickening agent and binder in paints, cosmetics, and similar products. Like other chlorinated h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |