|
Synthetic Radioisotope
A synthetic radioisotope is a radionuclide that is not found in nature: no natural process or mechanism exists which produces it, or it is so unstable that it decays away in a very short period of time. Examples include technetium-95 and promethium-146. Many of these are found in, and harvested from, spent nuclear fuel assemblies. Some must be manufactured in particle accelerators. Production Some synthetic radioisotopes are extracted from spent nuclear reactor fuel rods, which contain various fission products. For example, it is estimated that up to 1994, about 49,000 terabecquerels (78 metric ton) of technetium was produced in nuclear reactors, which is by far the dominant source of terrestrial technetium. Some synthetic isotopes are produced in significant quantities by fission but are not yet being reclaimed. Other isotopes are manufactured by neutron irradiation of parent isotopes in a nuclear reactor (for example, Tc-97 can be made by neutron irradiation of Ru-96) or b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uses Of Radioactivity In Oil And Gas Wells
Radioactive sources are used for logging formation parameters. Radioactive tracers, along with the other substances in hydraulic-fracturing fluid, are sometimes used to determine the injection profile and location of fractures created by hydraulic fracturing. Use of radioactive sources for logging Sealed radioactive sources are routinely used in formation evaluation of both hydraulically fractured and non-fracked wells. The sources are lowered into the borehole as part of the well logging tools, and are removed from the borehole before any hydraulic fracturing takes place. Measurement of formation density is made using a sealed caesium-137 source. This bombards the formation with high energy gamma rays. The attenuation of these gamma rays gives an accurate measure of formation density; this has been a standard oilfield tool since 1965. Another source is americium berylium (Am-Be) neutron source used in evaluation of the porosity of the formation, and this has been used since 1950 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively. Beta particles with an energy of 0.5 MeV have a range of about one metre in air; the distance is dependent on the particle energy. Beta particles are a type of ionizing radiation and for radiation protection purposes are regarded as being more ionising than gamma rays, but less ionising than alpha particles. The higher the ionising effect, the greater the damage to living tissue, but also the lower the penetrating power of the radiation. Beta decay modes β− decay (electron emission) An unstable atomic nucleus with an excess of neutrons may undergo β− decay, where a neutron is converted into a proton, an electron, and an electron antineutrino (the antip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) They are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
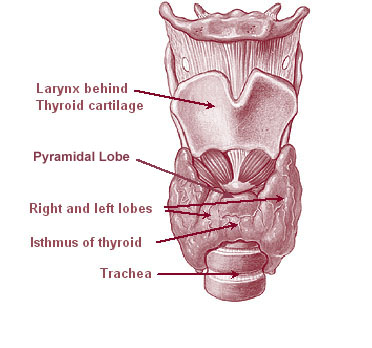
Thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The thyroid is located at the front of the neck, below the Adam's apple. Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones triiodothyronine (T3) and thyroxine (T4)and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis, and in children, growth and development. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unsealed Source Radiotherapy
Radionuclide therapy (RNT, also known as unsealed source radiotherapy or molecular radiotherapy) uses radioactive substances called radiopharmaceuticals to treat medical conditions, particularly cancer. These are introduced into the body by various means (injection or ingestion are the two most commonplace) and localise to specific locations, organs or tissues depending on their properties and administration routes. This includes anything from a simple compound such as sodium iodide that locates to the thyroid via trapping the iodide ion, to complex biopharmaceuticals such as recombinant antibodies which are attached to radionuclides and seek out specific antigens on cell surfaces. This is a type of targeted therapy which uses the physical, chemical and biological properties of the radiopharmaceutical to target areas of the body for radiation treatment. The related diagnostic modality of nuclear medicine employs the same principles but uses different types or quantities of radioph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide. Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about 584.3±1 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236. Natural decay chain :\begin \ce \begin \ce \\ \ce \end \ce \\ \ce \begin \ce \\ \ce \end \ce \end Fission properties The fission of one atom of uranium-235 releases () inside the reactor. That corresponds to 19.54 TJ/ mol, or 83.14 TJ/kg. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum-99
Molybdenum (42Mo) has 33 known isotopes, ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. All unstable isotopes of molybdenum decay into isotopes of zirconium, niobium, technetium, and ruthenium. Molybdenum-100 is the only naturally occurring isotope that is not stable. Molybdenum-100 has a half-life of approximately 1×1019 y and undergoes double beta decay into ruthenium-100. Molybdenum-98 is the most common isotope, comprising 24.14% of all molybdenum on Earth. Molybdenum isotopes with mass numbers 111 and up all have half-lives of approximately .15 s. List of isotopes , - , rowspan=2, 83Mo , rowspan=2 style="text-align:right" , 42 , rowspan=2 style="text-align:right" , 41 , rowspan=2, 82.94874(54)# , rowspan=2, 23(19) ms (+30-3) ms, β+ , 83Nb , rowspan=2, 3/2−# , rowspan=2, , rowspan=2, , - , β+, p , 82Zr , - , 84Mo , s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium-99m Generator
A technetium-99m generator, or colloquially a technetium cow or moly cow, is a device used to extract the metastable isotope 99mTc of technetium from a decaying sample of molybdenum-99. 99Mo has a half-life of 66 hours and can be easily transported over long distances to hospitals where its decay product technetium-99m (with a half-life of only 6 hours, inconvenient for transport) is extracted and used for a variety of nuclear medicine diagnostic procedures, where its short half-life is very useful. Parent isotope source 99Mo can be obtained by the neutron activation (n,γ reaction) of 98Mo in a high neutron flux reactor. However, the most frequently used method is through fission of uranium-235 in a nuclear reactor. While most reactors currently engaged in 99Mo production use highly enriched uranium-235 targets, proliferation concerns have prompted some producers to transition to low-enriched uranium targets. The target is irradiated with neutrons to form 99Mo as a fission pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma-ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nucleus, atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequency, frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Ulrich Villard, Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha particle, alpha rays and beta particle, beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |