|
Synthesis Gas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII (in Germany alone half a million cars were built or rebuilt to run on wood gas). Production Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of methane: : In principle, but rarely in practice, biomass and related hydrocarbon feedstocks could be used to generate biogas and biochar in waste-to-energy gasification facilities. The gas generated (mostly methane and carbon dioxide) is sometimes described as ''syngas'' but its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomass
Biomass is plant-based material used as a fuel for heat or electricity production. It can be in the form of wood, wood residues, energy crops, agricultural residues, and waste from industry, farms, and households. Some people use the terms biomass and biofuel interchangeably, while others consider biofuel to be a ''liquid'' or ''gaseous'' fuel used for transportation, as defined by government authorities in the US and EU. The European Union's Joint Research Centre defines solid biofuel as raw or processed organic matter of biological origin used for energy, such as firewood, wood chips, and wood pellets. In 2019, biomass was used to produce 57 EJ (exajoules) of energy, compared to 190 EJ from crude oil, 168 EJ from coal, 144 EJ from natural gas, 30 EJ from nuclear, 15 EJ from hydro and 13 EJ from wind, solar and geothermal combined. Approximately 86% of modern bioenergy is used for heating applications, with 9% used for transport and 5% for electricity. Most of the global b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-temperature Electrolysis
High-temperature electrolysis (also HTE or steam electrolysis) is a technology for producing hydrogen from water at high temperatures. Efficiency High temperature electrolysis is more efficient economically than traditional room-temperature electrolysis because some of the energy is supplied as heat, which is cheaper than electricity, and also because the electrolysis reaction is more efficient at higher temperatures. In fact, at 2500 °C, electrical input is unnecessary because water breaks down to hydrogen and oxygen through thermolysis. Such temperatures are impractical; proposed HTE systems operate between 100 °C and 850 °C. If one assumes that the electricity used comes from a heat engine, it takes 141.86 megajoules (MJ) of heat energy to produce one kg of hydrogen, for the HTE process itself and for the electricity required. At 100 °C, 350 MJ of thermal energy are required (41% efficient). At 850 °C, 225 MJ are required (64% efficient). Above 8 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Renewable Energy
Renewable energy is energy that is collected from renewable resources that are naturally replenished on a human timescale. It includes sources such as sunlight, wind, the movement of water, and geothermal heat. Although most renewable energy sources are sustainable, some are not. For example, some biomass sources are considered unsustainable at current rates of exploitation. Renewable energy often provides energy for electricity generation to a grid, air and water heating/cooling, and stand-alone power systems. Renewable energy technology projects are typically large-scale, but they are also suited to rural and remote areas and developing countries, where energy is often crucial in human development. Renewable energy is often deployed together with further electrification, which has several benefits: electricity can move heat or objects efficiently, and is clean at the point of consumption. In addition, electrification with renewable energy is more efficient and therefore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
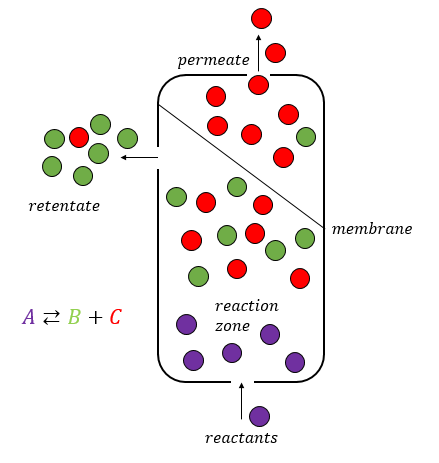
Membrane Reactor
A membrane reactor is a physical device that combines a chemical conversion process with a membrane separation process to add reactants or remove products of the reaction. Chemical reactors making use of membranes are usually referred to as membrane reactors. The membrane can be used for different tasks: * Separation ** Selective extraction of products ** Retention of the catalyst * Distribution/dosing of a reactant * Catalyst support (often combined with distribution of reactants) Membrane reactors are an example for the combination of two unit operations in one step, e.g., membrane filtration with the chemical reaction. The integration of reaction section with selective extraction of a reactant allows an enhancement of the conversions compared to the equilibrium value. This characteristic makes membrane reactors suitable to perform equilibrium-limited endothermic reactions. Benefits and critical issues Selective membranes inside the reactor lead to several benefits: reactor sec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine Scrubbing
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from gases. It is a common unit process used in refineries, and is also used in petrochemical plants, natural gas processing plants and other industries. Processes within oil refineries or chemical processing plants that remove hydrogen sulfide are referred to as "sweetening" processes because the odor of the processed products is improved by the absence of hydrogen sulfide. An alternative to the use of amines involves membrane technology. However, membrane separation is less attractive due to the relatively high capital and operating costs as well as other technical factors. Many different amines are used in gas treating: * Diethanolamine (DEA) * Monoethanolamine (MEA) * Methyldiethanolamine (MDEA) * Diisopropanol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure Swing Adsorption
Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases (typically air) under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperature and significantly differs from the cryogenic distillation commonly used to separate gases. Selective adsorbent materials (e.g., zeolites, (aka molecular sieves), activated carbon, etc.) are used as trapping material, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed gas. Process Pressure swing adsorption process (PSA) is based on the phenomenon that under high pressure, gases tend to be trapped onto solid surfaces, ''i.e.'', to be "adsorbed". The higher the pressure, the more gas is adsorbed. When the pressure is dropped, the gas is released, or desorbed. PSA can be used to separate gases in a mixture because different gases are adsorbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water-gas Shift Reaction
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer okewith air and gasifying it with steam". The caloric yield of this is about 10% of a modern syngas plant. Further making this technology unattractive, its precursor coke is expensive, whereas syngas uses cheaper precursor, mainly methane from natural gas. Production Synthesis gas is made by passing steam over a red-hot carbon fuel such as coke: : (ΔH = +131 kJ/mol) The reaction is endothermic, so the fuel must be continually re-heated to maintain the reaction. To do this, an air stream, which alternates with the vapor stream, is introduced to combust some of the carbon: : (ΔH = -393 kJ/mol) Theoretically, to make 6 L of water gas, 5 L of air is required. Alternatively, to prevent contamination with nitrogen, energy can be provided by using pure oxygen to burn carbon into carbon monoxide. : (ΔH = -221 kJ/mol) In this case, 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change Δ''G''⚬ is negative." A strongly exothermic reaction will usually also be exergonic because Δ''H''⚬ makes a major contribution to Δ''G''⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Examples Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction: :4Fe + 3O2 → 2Fe2O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Gas
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer okewith air and gasifying it with steam". The caloric yield of this is about 10% of a modern syngas plant. Further making this technology unattractive, its precursor coke is expensive, whereas syngas uses cheaper precursor, mainly methane from natural gas. Production Synthesis gas is made by passing steam over a red-hot carbon fuel such as coke: : (ΔH = +131 kJ/mol) The reaction is endothermic, so the fuel must be continually re-heated to maintain the reaction. To do this, an air stream, which alternates with the vapor stream, is introduced to combust some of the carbon: : (ΔH = -393 kJ/mol) Theoretically, to make 6 L of water gas, 5 L of air is required. Alternatively, to prevent contamination with nitrogen, energy can be provided by using pure oxygen to burn carbon into carbon monoxide. : (ΔH = -221 kJ/mol) In this case, 1&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon dioxide, nitrogen, hydrogen sulfide, and helium are also usually present. Natural gas is colorless and odorless, so odorizers such as mercaptan (which smells like sulfur or rotten eggs) are commonly added to natural gas supplies for safety so that leaks can be readily detected. Natural gas is a fossil fuel and non-renewable resource that is formed when layers of organic matter (primarily marine microorganisms) decompose under anaerobic conditions and are subjected to intense heat and pressure underground over millions of years. The energy that the decayed organisms originally obtained from the sun via photosynthesis is stored as chemical energy within the molecules of methane and other hydrocarbons. Natural gas can be burned fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Density
In physics, energy density is the amount of energy stored in a given system or region of space per unit volume. It is sometimes confused with energy per unit mass which is properly called specific energy or . Often only the ''useful'' or extractable energy is measured, which is to say that inaccessible energy (such as rest mass energy) is ignored. In cosmological and other general relativistic contexts, however, the energy densities considered are those that correspond to the elements of the stress–energy tensor and therefore do include mass energy as well as energy densities associated with pressure. Energy per unit volume has the same physical units as pressure and in many situations is synonymous. For example, the energy density of a magnetic field may be expressed as and behaves like a physical pressure. Likewise, the energy required to compress a gas to a certain volume may be determined by multiplying the difference between the gas pressure and the external pressure by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |