|
Surface Core Level Shift
A surface core level shift (SCS) is a kind of core-level shift that often emerges in X-ray photoelectron spectroscopy spectra of surface atoms. Because surface atoms have different chemical environments from bulk atoms, small shifts of binding energies are observed by X-ray photoelectron spectroscopy. SCS is ascribed mainly to the lower coordination numbers of surface atoms than bulk atoms. Reduced coordination leads to narrower valence Valence or valency may refer to: Science * Valence (chemistry), a measure of an element's combining power with other atoms * Degree (graph theory), also called the valency of a vertex in graph theory * Valency (linguistics), aspect of verbs rel ... bandwidth. Such narrowing of the bandwidth increases the density of states, and if more than half of the valence band is filled, the band center is lower than bulk and the binding energy increases. In contrast, if less than half of the valence band is filled, the band center is higher than bulk, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Core-level
Core electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play a secondary role in chemical bonding and reactions by screening the positive charge of the atomic nucleus from the valence electrons. The number of valence electrons of an element can be determined by the periodic table group of the element (see valence electron): *For main group elements, the number of valence electrons ranges from 1-8 electrons (''n''s and ''n''p orbitals). *For transition metals, the number of valence electrons ranges from 3-12 electrons (''n''s and (''n''−1)d orbitals). *For lanthanides and actinides, the number of valence electrons ranges from 3-16 electrons (''n''s, (''n''−2)f and (''n''−1)d orbitals). All other non-valence electrons for an atom of that element are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
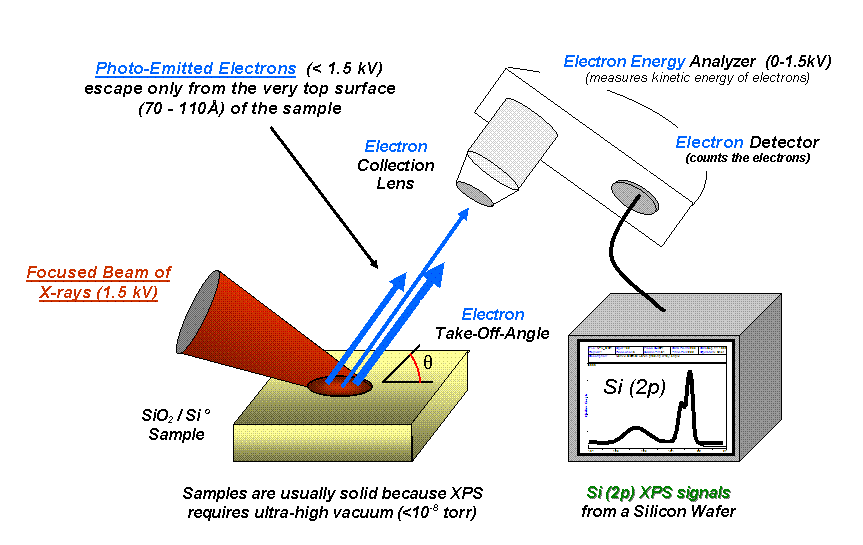
X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material (elemental composition) or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation. XPS belongs to the family of photoemission spectroscopies in which electron population spectra are obtained by irr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals. For molecules and polyatomic ions the coordination number of an atom is determined by simply counting the other atoms to which it is bonded (by either single or multiple bonds). For example, r(NH3)2Cl2Br2sup>− has Cr3+ as its central cation, which has a coordination number of 6 and is described as ''hexacoordinate''. The common coordination numbers are 4, 6 and 8. Molecules, polyatomic ions and coordination complexes In chemistry, coordination number, defined originally in 1893 by Alfred Werner, is the total number of neighbors of a central atom in a molecule or ion. The concept is most commonly applied to coordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantizat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |